As the Jack Fowler Professor of the University of Wisconsin Department of Human Oncology, Principal Investigator for the Wisconsin H&N SPORE Grant and member of the UW Carbone Cancer Center Senior Leadership Council, I facilitate the interaction of investigators involved in basic, translational and clinical cancer research activities. I am deeply committed to mentorship of trainees and leadership of multidisciplinary clinical, teaching and research teams.
The overall theme of my research program is to improve treatment outcome for patients with head and neck cancer (HNC). Areas of particular research emphasis include the interaction of molecular growth inhibitors combined with radiation and the use of conformal radiation treatment techniques to limit normal tissue toxicity. I recently served a four-year term on the Presidential Track of ASTRO (2016-2020) and thereafter as President of the American Radium Society (2023-2024). I serve as Principal Investigator for the UW Head and Neck Cancer SPORE Grant, a $15 million dollar effort (2022-2027) to improve outcomes in HNC. We are among four centers in the nation currently with a NIH HN SPORE Grant. I have been an active RTOG/NRG HNC Committee member for 28 years and serve as PI or Co-PI for a series of national and international clinical trials that have changed global practice for patients with HNC.
I served for 17 years as Chairman of the Department of Human Oncology at the University of Wisconsin (2007-2024) and am very proud of the high-quality faculty, staff and trainees who work tirelessly on behalf of cancer patient care and cancer research each day. Our teams illuminate the power and precision of radiation to heal, to cure, to image, to improve human health and quality of life. A unique and highly appealing aspect of radiation oncology is the opportunity for close collaboration between technology, biology and clinical medicine. Never before have the prospects for impactful advances in our field been so bright, and many of these advances involve multi-investigator teams, working together to achieve new milestones.
Dr. Harari's UW Health ProfileEducation
Resident, University of Arizona, Radiation Oncology (1990)
Intern, University of California-Davis, Internal Medicine (1985)
MD, University of Virginia, Medicine (1984)
BS, Tufts University, Biology (1980)
Academic Appointments
Jack Fowler Professor and Chairman, Human Oncology (2007-2024)
Associate Director, University of Wisconsin Carbone Cancer Center, University of Wisconsin Carbone Cancer Center (2007)
Jack Fowler Professor (Endowed Professorship), Human Oncology (2003)
Associate Professor, Human Oncology (1999)
Assistant Professor (tenure track), Human Oncology (1993)
Selected Honors and Awards
President, American Radium Society (2023-2024)
ASTRO Presidential Track (2016-2020)
PI, NIH H&N Cancer SPORE Grant at the University of Wisconsin (2016–pres.)
Canadian Association Radiation Oncology (CARO) Lecturer (2014)
Katie Dinshaw Annual Oration, Indian Cancer Congress (2013)
RSNA Annual Oration in Radiation Oncology (2013)
Wharton Lecturer, Princess Margaret Hospital (2011)
ASTRO Fellow Inductee (FASTRO) (2011)
Alpha Omega Alpha Alumnus Inductee, University of Virginia School of Medicine (2011)
Course Completion: Program for Chiefs of Clinical Services, Harvard School of Public Health (2009)
ESTRO Honorary Membership Award (2009)
Harold P. Rusch Award for Translational Cancer Research (2005)
Jack Fowler Endowed Professorship in Human Oncology (2003–pres.)
Academic Sabbatical, Peter MacCallum Cancer Institute, Melbourne, Australia (2001)
Wisconsin/Hilldale Faculty/Undergraduate Research Award (1998)
Teacher of the Year in Radiation Oncology, University of Wisconsin (1998)
Wisconsin/Hilldale Faculty/Undergraduate Research Award (1995)
NIH First Award, R-29 (1995)
ASTRO Resident Research Award presented to Radiation Oncology Resident (D. Petereit), for work carried out in P. Harari laboratory during ASTRO fellowship year (1993)
RSNA Scholars Program Grant (1993)
American Cancer Society Career Development Award (1992)
Teacher of the Year in Radiation Oncology, University of Wisconsin (1991)
International Cancer Research Technology Transfer (ICRETT) Fellowship to study at Mount Vernon Hospital and Gray Laboratory, London, England (1990)
Resident Essay Award, American Society for Therapeutic Radiology and Oncology (ASTRO) (1989)
American Radium Society Young Oncologist Travel Grant for Radiation Oncology (1989)
Junior Investigator Travel Award for the Sixth International Conference on Chemical Modifiers of Cancer Treatment. Paris, France (1988)
Postdoctoral NIH Fellowship Grant under E.W. Gerner, Ph.D., for Cancer Biology Research (1986)
R.H. Love Scholarship for Medical Study (1983)
Society of the Cincinnati Scholarship for Academic Achievement (1982)
Lawson Memorial Scholarship for Medical Study (1981)
Paul A. Warren Scholarship for Excellence in Genetics (1980)
Magna Cum Laude graduate, B.S. Biology, Tufts University (1980)
Phi Beta Kappa (1979)
Boards, Advisory Committees and Professional Organizations
ASTRO Board of Directors, Presidential Track (2016–pres.)
ASTRO Board of Directors, Education Council (2016–2020)
NCI Head and Neck Cancer Steering Committee (2010–pres.)
ASTRO Head and Neck Resource Panel (2010–pres.)
Chair Search Committee, UW Department of Medical Physics (2011–2013)
UW Hospital Department Chairman Leadership Group (2010–pres.)
ASTRO Strategic Planning Task Force (2010)
ASTRO Red Journal Editor Selection Task Force (2010)
ASTRO Practical Radiation Oncology Journal Editor Selection Task Force (2009)
Scientific Committee, Wolfsberg Meeting on Molecular Radiation Biology/Oncology (2011–pres.)
UW School of Medicine Council of Chairs (2007–pres.)
ASTRO Annual Meeting Scientific Program Committee (2005–2010)
American H&N Society, Annual Meeting Program Committee (2009–2010)
ASTRO CME/MOC Committee of the Education Council (2007–2010)
Immediate Past Chair, ASTRO Education Committee of the Education Council (2009)
Program Committee, American Head and Neck Society (2007–2008)
ASTRO Research Evaluation Committee of the Research Council (2007–2011)
Associate Director, University of Wisconsin Carbone Comprehensive Cancer Center (2007–2016)
Chairman, 1st ASTRO/ASCO/AHNS Multidisciplinary H&N Cancer Symposium (2006–20007)
ASTRO Meeting Program Committee (2005–2007)
Chairman, ASTRO Education Committee (2004–2008)
ASCO Program Committee (2004–2008)
UWCCC Clinical Research Committee (2003–2008)
American Radium Society, Executive and Program Committee (2002–2006)
ASCO Education Committee, Head & Neck Track Leader (2002–2004)
ASCO Grants Selection Committee (2002–2004)
ASTRO Education Committee (2001–2004)
NCI Signal Transduction Working Group (2001–2007)
University of Wisconsin Comprehensive Cancer Center Scientific Review Committee (1999–pres.)
Residency Training Program Director, University of Wisconsin, Radiation Oncology (1997–2007)
1998–2000 University of Wisconsin Medical Foundation Long Range Planning Committee (1998–2000)
American College of Radiology, Committee on Residency Training in Radiation Oncology of the Commission on Education (1996–2007)
Medical Director, University of Wisconsin Radiation Oncology Quality Assurance Program (1995–1998)
University of Wisconsin, PI for Radiation Therapy Oncology Group (RTOG) (1994–2007)
Radiation Therapy Oncology Group (RTOG), Head and Neck Committee and Time/Dose/Volume Committee Co-Chair (1992–1996)
Eastern Cooperative Oncology Group (ECOG), Head and Neck Committee Radiotherapy Co-Chair (1992–1994)
Program Committee, 4th Research Conference on the Biology, Treatment and Prevention of Head and Neck Cancer (1992–1994)
National Cancer Institute, Head and Neck Cancer Strategy Committee (1992–2004)
University of Wisconsin Medical Foundation Compensation and Benefit Task Force (1995–1996)
Department of Human Oncology and UWCCC Cancer Registry Data Committee (1992–2004)
Associate, University of Wisconsin Center for Tobacco Research and Intervention (1992–2010)
University of Wisconsin Comprehensive Cancer Center Clinical Affairs Committee (1992–1995)
University of Wisconsin, Department of Human Oncology Research and Development Committee (1994–pres.)
Research Focus
Head and Neck Oncology, Molecular Modulation of Radiation Response, Conformal Head and Neck Radiation Treatment Techniques, Molecular Inhibition of Growth Factor Receptor Signaling
Dr. Paul Harari is the Jack Fowler Professor in the Department of Human Oncology, principal investigator for the Wisconsin H&N SPORE Grant and member of the UW Carbone Cancer Center Senior Leadership Council. His clinical and laboratory research is focused on improving treatment outcomes for patients with head and neck cancer.
Harari Lab
Head and Neck Cancer Specialized Program of Research Excellence (HN SPORE)
Dr. Harari’s research program focuses on translating important discoveries from laboratory to clinical application with the ultimate goal to improve diagnosis, treatment and quality of life for head and neck cancer (HNC) patients. Dr. Harari is the Principal Investigator for the $15M HNC SPORE Grant at the University of Wisconsin, which involves over 40 faculty members across the School of Medicine and Public Health, Carbone Cancer Center and beyond. This NIH award includes 4 primary scientific translational Research Projects, 3 Cores (Admin, Pathology/Biospecimen Core, and Biostatistics and Bioinformatics Core) and 2 pilot funding programs that award approximately $400,000 per year to innovative HNC research investigators, both seasoned as well as early career investigators. This prestigious SPORE Grant is the first of its kind to be awarded in the State of Wisconsin.
The ultimate objective of the Wisconsin Head and Neck (HN) SPORE is to improve overall outcomes for HNC patients. The Wisconsin HN SPORE Team commenced formal function in July 2010 and established three primary missions: 1) promote systematic collaboration between UW basic scientists and clinicians in the discipline of HN oncology, 2) prepare the highest quality SPORE application possible to promote HN oncology translational research reflecting the scientific and clinical expertise at the University of Wisconsin, and 3) make a meaningful and lasting impact in the treatment and outcome for patients with HN malignancies. The first SPORE Award in the State of Wisconsin was earned with an overall Impact Score of 1.5 and commenced funding in 2016. The strong and sustained collaboration between basic scientists and HNC clinicians remains the foundation of the Wisconsin HN SPORE.

The UW HNC SPORE exploits several scientific and clinical strengths, specific to the UW.
Cancer Virology: The University of Wisconsin-Madison is fortunate to have one of the preeminent Human Cancer Virology Programs in the world, deriving largely from the McArdle Cancer Research Laboratory and the Institute for Molecular Virology. Two accomplished virologists and long-time collaborators, Dr. Paul Lambert (NCI Outstanding Investigator, Howard M. Temin Professor and Chair of Oncology, McArdle Laboratory for Cancer Research Director) and Dr. Paul Ahlquist (Howard Hughes Medical Institute Investigator, National Academy of Sciences Member) collaborate on Project 1 along with Co-Leader, Dr. Timothy McCulloch, Professor and Chair of the Department of Surgery, Otolaryngology. This collaboration has contributed to the excellent productivity of the current Project 1 and evolution into the new Project 1 proposed for the competitive SPORE renewal. In addition, Dr. Ahlquist serves as a mentor for two SPORE CEP recipients, Dr. Tony Gitter and Dr. Irene Ong, both of whom have pursued development of new computational methods for subtyping HNC based upon combinatorial interrogation of deep seq data sets. Dr. Lambert serves as Associate Director of the SPORE and advisor to the Path Core. The multimodal involvement of these world class cancer virologists in the SPORE provides important basic science expertise that informs scientific development across several SPORE projects and cores.
Radiation Oncology: The University of Wisconsin HN SPORE exploits a long tradition of research and clinical excellence in Radiation Oncology and Radiobiology led by Dr. Paul Harari, department chairman and overall PI for the Wisconsin HN SPORE. The capacity of radiation to enhance immune reactivity of HN tumors is developed by physician scientist Dr. Zach Morris in Projects 2 and 3. In fact, radiation expertise is brought to bear across several SPORE projects in which radiation is combined with drug therapy in animal model systems and in human clinical trials (Projects 2, 3 and 4). Project 2 advances a novel radionuclide molecule synthesized and developed at Wisconsin (Dr. Weichert, molecule HN600) for translational and clinical trial testing, and Project 4 brings forth a powerful patient-derived xenograft model that can test radiation and drug interactions with potential predict treatment response for HNC patients. Multiple projects and specific aims directly engage radiation questions in light of the central role radiation plays in the treatment of HNC.
HNC Biospecimen Resources: The Wisconsin HN SPORE Program, in close collaboration with the University of Wisconsin Carbone Cancer Center (UWCCC), has developed one of the most robust and high quality biospecimen repositories in the world for the study of HNC. Critical reagents and specimens include; formalin-fixed paraffin embedded (FFPE) HNC and adjacent normal tissues, frozen HNC and adjacent normal tissues, HNC tissue microarray (TMAs), HNC patient derived xenografts (PDXs), HPV-validated HNC cell lines, blood components from HNC patients, among others. The HN SPORE Path Core is fully integrated and coordinated with the UWCCC Translational Science BioCore and is co-housed within the same laboratory space. Faculty leadership from the Department of Pathology overlaps between the UWCCC and the HN SPORE Path Core providing optimal integration and cooperation for these critically important resources.
Translational Human Endpoint Strategies: Over the last several years, we have significantly advanced our development of specialized human tissue microarrays (TMAs) in HNC for use across several projects. These fully annotated TMAs provide a powerful resource for targeted biomarker discovery and validation within Project 1. These studies also provide classic examples of translational research in the “reverse” direction, using human biospecimens, from clinical trials in some cases, to study new biological phenomena, optimize previous findings, and develop new hypotheses based on results obtained from human studies and tissues.
Projects 2 and 4 culminate in phase I clinical trials that examine the incorporation of a novel radiolabeled molecule initially developed at the University of Wisconsin, CLR 131, in recurrent HNC (project 2) and investigate the role of AXL to modulate treatment response in HNC (project 4). Project 3 includes an early phase II study which utilizes radiation therapy to help the patient’s immune system better recognize their cancer and combines this with immune stimulating treatments to enable the patient’s immune system to destroy tumor cells anywhere in the patient’s body. Two of the projects (Projects 1 and 4) employ high throughput molecular analysis of gene expression in HNC tumor models using the newly established human patient-derived xenograft system. Two of the projects (Projects 1 and 4) study detailed signal transduction pathways for which we have powerful basic science expertise (HPV, syndecan and EGFR/HER biology respectively) that will be leveraged towards new biomarker identification and future drug target development.
Wisconsin HN SPORE Organizational Chart
Description of HN SPORE Research Projects:
HN SPORE PROJECT 1: Defining and Targeting Pathways that Drive H&N Cancer (Lambert, McCulloch)
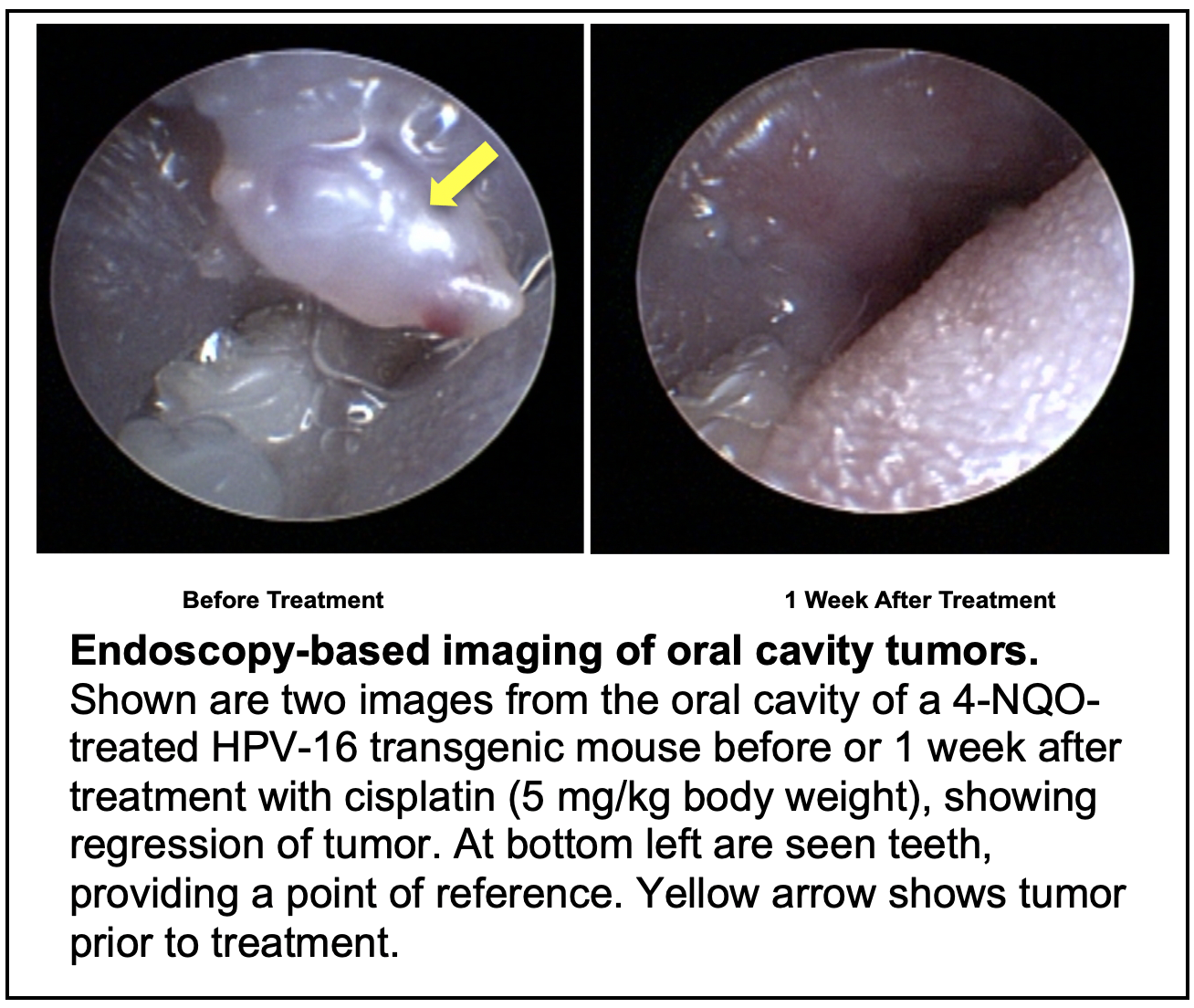
In this project, new HPV+ and HPV- mouse models for HNC will be utilized to define mutations that drive HNC development. Mutation selective therapy approaches will be investigated using the Wisconsin HN patient-derived xenograft model system. Biomarkers that predict disease severity and response to therapy will be evaluated for their prognostic value using human HNC tissue microarrays.
SIGNIFICANCE: Recent exome/seq analyses of human HNCs have identified p53, p16 and Notch genes as potential tumor suppressors of HNC and PI3K as a potential oncogene in HNC. Of these only Notch and PI3K are commonly found mutated in HPV+ HNC, consistent with the fact that HPV oncogenes E6 and E7 disrupt the p53 and the pRb/p16 pathways, respectively. We will interrogate the importance of mutations in these genes/pathways in driving HPV+ versus HPV- HNC using available Genetically Engineered Mouse (GEM) strains. Preliminary data suggests that Notch alone or together with HPV oncogenes serves as a driver of head and neck carcinogenesis, consistent with the hypothesizes put forth in this project. Additional preliminary data indicate the feasibility of pursuing studies on the role of Notch and p53 in HPV- HNCs. We will use genome wide analyses to identify other genes/pathways altered in HNC that could also drive HNCs and test them using available or to-be-generated GEM models. Finally, we will attempt to develop the first-ever mouse model for tobacco-driven HNC, and, if successful, employ it in the above-described studies.
TRANSLATIONAL RELEVANCE: In this project we make use of our prior expertise in establishing preclinical, mouse models for HNCs to identify driver mutations in these cancers and evaluate the importance of specific genes as therapeutic targets in treating HPV+ vs HPV- HNC. Genome wide analyses of these mouse models will be used to identify biomarkers that are predictive of disease severity and response to therapy in human HNCs. Using human patient-derived xenografts and well-annotated human HNC tissue microarrays, we will perform human endpoint analyses to identify prognostic biomarkers that predict tumor response of individual HNC patients to the novel clinical treatment strategies being tested in this project.
PROJECT 2: Therapeutic Combination of CLR 131 with External Beam Radiation in HNC (Harari, Hartig)
This project will advance a promising new imaging and cancer therapy molecule (CLR 131) developed at UW for the treatment of HNC patients. A fully automated Monte Carlo dosimetry platform for tumor dose estimation will be established to personalize radiation dose for human studies. This project will culminate in a phase I clinical trial of CLR 131 combined with reduced-dose external beam radiation in recurrent HNC patients.
SIGNIFICANCE: Although significant technical advances have been made in the delivery and dose shaping of radiation with the advent of 3D-conformal and intensity modulated radiation therapy (IMRT), the maximal tolerated dose remains limited by normal tissue toxicity. A promising novel approach to solving this problem is to deliver radiation within the tumor using a biological method that employs a radiolabelled drug that accumulates within tumors and thus offers “internal radiation,” thereby limiting dose to surrounding normal tissues. This would allow for a decrease in dose applied via external beam radiation, thereby reducing side effects, while maintaining or potentially increasing tumor control. Such an approach is of particular significance in HNC because surgery and radiation often compromise normal salivary and swallow function with a powerful adverse impact on patient QOL. The proposed research will develop and advance translational research with this unique radiolabelled diapeutic compound to serve this critical need. The successful use of CLR 131 in HNC is significant in specifically targeting tumors within its complex environment while sparing normal tissue. This project is highly significant because it presents the first translational steps toward this goal.
Within the spectrum of toxicities that occur following HNC radiation, altered salivary and swallow function are among the most prevalent and debilitating. Although the advent of conformal radiation treatment techniques has had a positive impact on QOL for HNC patients with reduction in dose to salivary glands, improvements are not universal and many patients still report significant xerostomia (dry mouth) and associated deficits in swallowing function and QOL. Accordingly, the development of new approaches that limit long term toxicities of external beam radiation are necessary and important to optimize HNC patient survival and QOL. The potential benefit of reducing radiation dose to other normal HN structures including other salivary structures and muscles directly involved in swallow mechanics and salivation may also be critical. Such dose reduction and sparing of high dose to HN normal tissues will be pursued in this study with CLR 131.
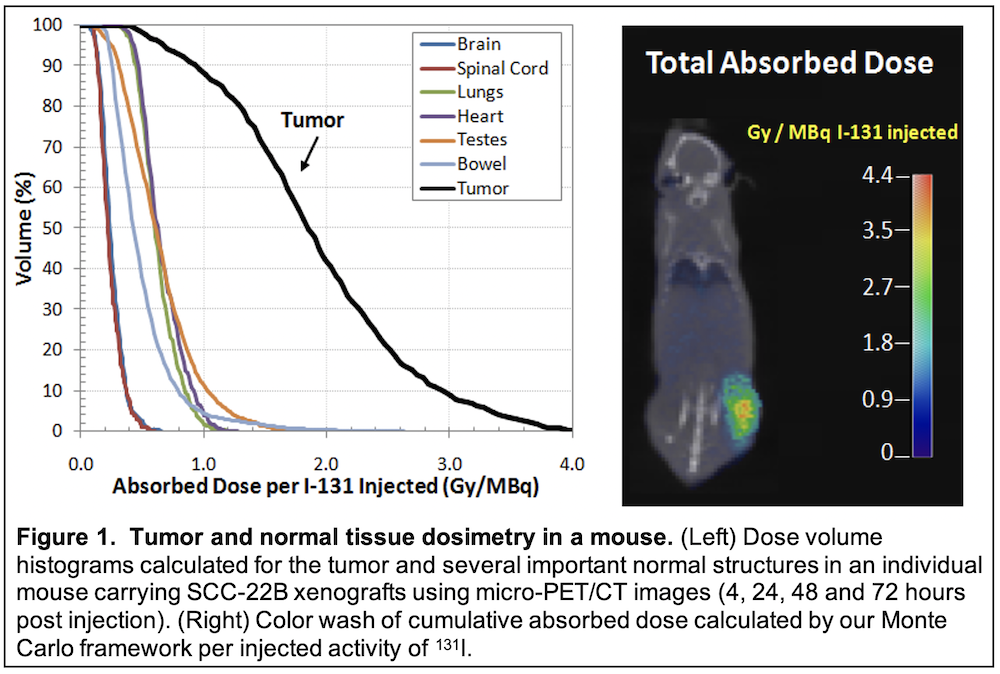
In the current proposal, we will test a promising new radiolabeled molecule (CLR 131) developed over the last decade at the University of Wisconsin. CLR 131 provides the opportunity for tumor-specific internal delivery of radiation thereby enabling combination with reduced dose external beam radiation in the treatment of recurrent HNC. This agent, which is radiolabeled with 131-I, shows selective accumulation and retention in human tumors across a broad spectrum of animal models (over 50 to date) including our HNC patient-derived xenografts.
TRANSLATIONAL RELEVANCE: We will first investigate the capacity of CLR 131 to accumulate and retain in tumors following systemic administration in HNC animal model systems. This will include detailed evaluation of dose deposition in mice harboring human HN tumors xenografts following treatment with radiolabeled CLR1 131. Thereafter, we will investigate use of combined CLR 131 and external beam radiation to improve tumor control over that achievable with external beam radiation alone. This will provide experimental proof that radiolabeled CLR 131 augments tumor response to external beam radiotherapy. Finally, we will perform a phase I clinical trial to examine the combination of CLR 131 plus external beam radiation in patients with loco-regional recurrence in previously irradiated HN regions. Clinical endpoints will include feasibility, toxicity, tumor response, CLR 131 tumor uptake and HNC-specific QOL evaluation. Knowledge created by this work will test the approach of combining internal radiation with CLR 131 in HNC retreatment using a unique approach that limits normal tissue toxicities and adverse impact on QOL.
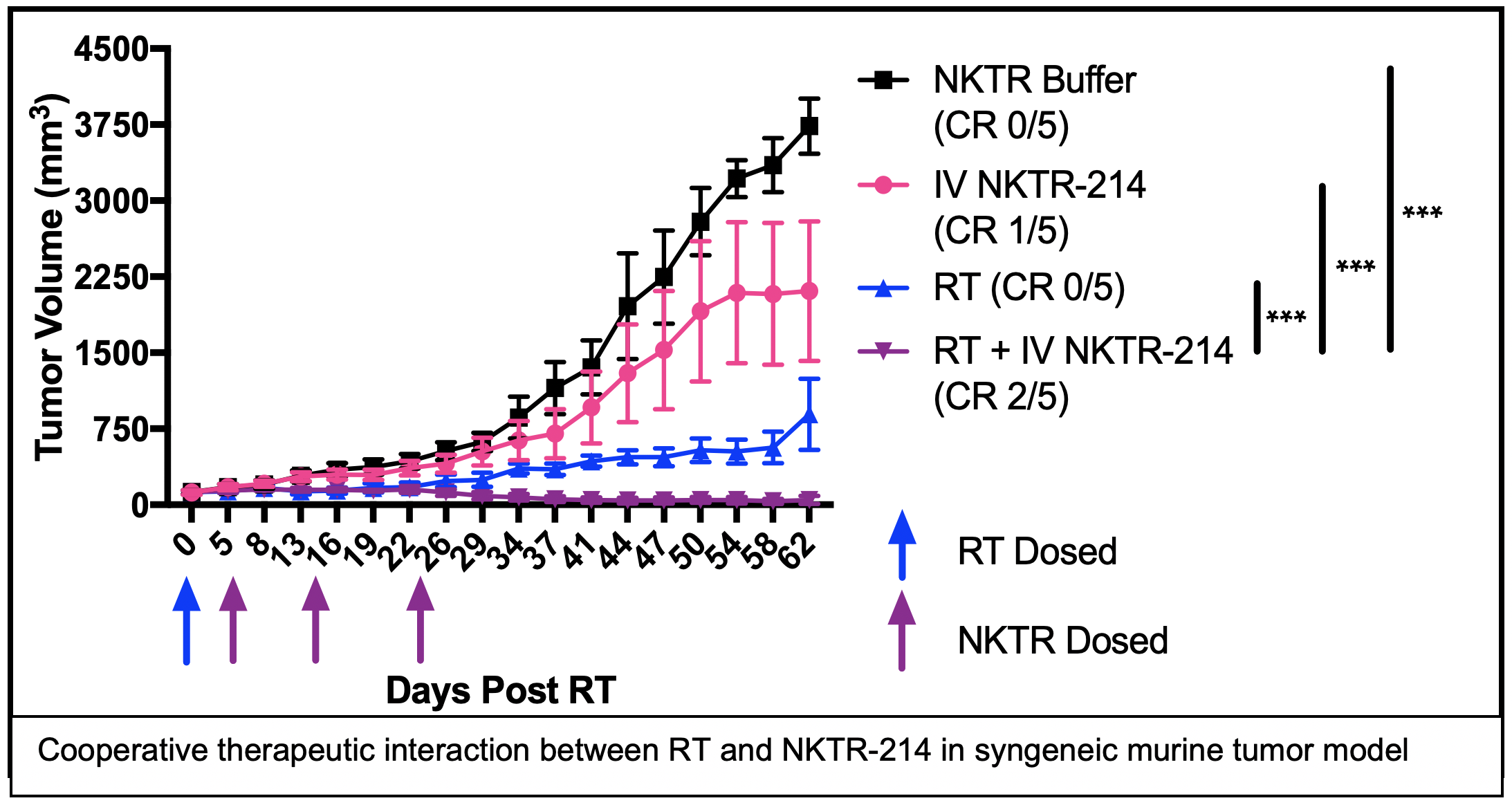
PROJECT 3: Combining radiation therapy and NKTR-214 to elicit in situ tumor vaccination in HNC (Morris, Harari)
Project 3 develops a new approach for the treatment of metastatic or recurrent head and neck cancers, utilizing radiation therapy to help the patient’s immune system to better recognize their cancer and combining this with immune stimulating treatments to enable the patient’s immune system to destroy tumor cells anywhere in the patient’s body. This combined treatment approach will be tested in mice and in a clinical trial in HNC patients in order to determine how the treatment works and to evaluate its safety and effectiveness.
SIGNIFICANCE: We aim to improve the cure rate for metastatic and recurrent HNC. To achieve this, we propose a combined modality approach to stimulate and diversify an endogenous anti-tumor immune response that is capable of recognizing and destroying metastatic tumors in a manner that will prevent recurrence and enable long-term cancer free survival. We hypothesize that our approach will overcome challenges that limit the efficacy of immune checkpoint inhibitors (ICI; e.g. anti-PD-1) in HNC. ICIs are a class of immunotherapies that modulate immune tolerance of a tumor by blocking specific inhibitory receptor-ligand interactions on the surface of T cells and thereby overcoming T cell inhibition or exhaustion. In patients with immunologically “hot” tumors, characterized by a pre-existing but exhausted anti-tumor immune response, anti-PD-1 can restore efficacy to the anti-tumor immune response, sometimes resulting in complete and durable regression of metastatic disease. However, ICIs have not shown clinical benefit in the treatment of immunologically “cold” cancers that are characterized by low levels of T cell infiltrate and/or low mutation burden resulting in few mutation-created neo-antigens. To improve the extent and duration of response to ICIs in HNC patients with immunologically “hot” tumors and to initiate a de novo anti-tumor immune response in HNC patients with “cold” tumors, we propose to combine systemic delivery of anti-PD-1 ICI with radiation therapy (RT) and PEGylated IL2 (NKTR-214) to elicit and propagate an in situ tumor vaccination.
This project will use syngeneic murine models of HNC to systematically optimize the capacity of RT + NKTR-214 to elicit in situ vaccine and to evaluate whether this combination may augment response to anti-PD-1 checkpoint blockade. We will further test whether response to this combination may be enhanced by addition of the anti-EGFR antibody, cetuximab, which may enhance antigen presentation via Fc-receptor-mediated engagement of antigen presenting cells.
TRANSLATIONAL RELEVANCE: In an early phase II clinical trial, we will test the safety of combining RT, NKTR-214, and anti-PD-1 therapies in patients with metastatic or recurrent HNC. Using blood and tumor tissue specimens from patients enrolled on this study, we will also evaluate for immunologic correlates of treatment response. The results derived from these studies should enable rapid translation of our findings to advanced phase clinical studies testing the efficacy of combining RT, NKTR-214, and anti-PD-1 therapies in patients with metastatic or recurrent HNC.
PROJECT 4: Role of receptor tyrosine kinase AXL in HNC therapy resistance (Wheeler, Kimple)
This project investigates the role of the receptor tyrosine kinase AXL in therapeutic resistance to the anti-EGFR antibody cetuximab. We recently identified that AXL plays a central role in cetuximab resistance as well as progression of HNSCC. In this application we will determine if 1) AXL mediates resistance by activating the HER3/PI3K/Akt axis via Src family kinases, 2) Determine if targeting AXL in mouse models of cetuximab resistance enhances tumor response to cetuximab and 3) Determine whether AXL predicts resistance to cetuximab in HNC patients and if targeting AXL in cetuximab-resistant PDXs enhances tumor response to cetuximab therapy.
 SIGNIFICANCE: Cetuximab resistance is a significant obstacle to the successful management of many HNC patients. Identifying novel strategies to predict and overcome cetuximab resistance in HNC is of high significance. In this application we have identified AXL, a novel receptor tyrosine kinase that drives resistance to cetuximab therapy. The proposed studies will establish the potential therapeutic benefit of simultaneously targeting both AXL and the EGFR signaling node. This will be undertaken by using anti-AXL antibody in combination with cetuximab. Successful pursuit of these investigations holds potential to significantly improve and refine current EGFR-centric therapeutic approaches in HNC and offer rapid translation of results to the clinic.
SIGNIFICANCE: Cetuximab resistance is a significant obstacle to the successful management of many HNC patients. Identifying novel strategies to predict and overcome cetuximab resistance in HNC is of high significance. In this application we have identified AXL, a novel receptor tyrosine kinase that drives resistance to cetuximab therapy. The proposed studies will establish the potential therapeutic benefit of simultaneously targeting both AXL and the EGFR signaling node. This will be undertaken by using anti-AXL antibody in combination with cetuximab. Successful pursuit of these investigations holds potential to significantly improve and refine current EGFR-centric therapeutic approaches in HNC and offer rapid translation of results to the clinic.
TRANSLATIONAL RELEVANCE: Preliminary data suggests that HNC patient-derived xenografts that express elevated levels of AXL predict resistance to cetuximab. In this application we will take advantage of a window-of-opportunity clinical trial to assess if high expression of AXL predicts resistance to cetuximab in HNC patients. From this patient data we will then develop patient-derived xenografts directly from patients with known cetuximab resistance. These tumors will be tested to determine if AXL blockade can re-sensitize tumors to cetuximab. Findings stemming from this work have the potential to shift future treatment practice paradigms by increasing our ability to predict those patients most likely to benefit from cetuximab and thereby personalize treatment approaches in advanced HNC.
HN SPORE 2019 Retreat
The University of Wisconsin Department of Human Oncology and the UW Carbone Cancer Center, under the direction of Principal Investigator Paul Harari, were awarded Wisconsin’s first NIH-funded Specialized Program of Research Excellence (SPORE) in August 2019.
The Wisconsin SPORE focuses on Head and Neck Cancer translational research, which means that the goals of each SPORE research project must link laboratory research (bench) to the clinic setting (bedside) and ultimately aim to improve overall outcome for patients with Head and Neck Cancer. The Wisconsin Head and Neck SPORE comprises four main translational research projects, three supporting cores and two pilot funding programs.



Publications Acknowledging Support from the Wisconsin H&N SPORE Grant 2016-present
- On-tissue derivatization with Girard’s reagent P enhances N-glycan signals for formalin-fixed paraffin-embedded tissue sections in MALDI mass spectrometry imaging Zhang H, Shi X, Vu NQ, Li G, Li Z, Shi Y, Li M, Wang B, Welham NV, Patankar MS, Weisman P, Li L;
Anal Chem. 2020. Epub 2020/09/01. doi: 10.1021/acs.analchem.0c02704. PMID: 32865977 - Cox regression with survival-time-dependent missing covariate values Yi Y, Ye T, Yu M, Shao J;
Biometrics. 76(2):460-71, 2020. Epub 2019/09/25. doi: 10.1111/biom.13155. PMID: 31549744; PMC7145010. - A PI3K/AKT Scaffolding Protein, IQ Motif-Containing GTPase Associating Protein 1 (IQGAP1), Promotes Head and Neck Carcinogenesis Wei T, Choi S, Buehler D, Anderson RA, Lambert PF;
Clin Cancer Res. 26(1):301-11, 2020. Epub 2019/10/11. doi: 10.1158/1078-0432.CCR-19-1063. PMID: 31597661; PMC6942630. - An Infection-Based Murine Model for Papillomavirus-Associated Head and Neck Cancer Wei T, Buehler D, Ward-Shaw E, Lambert PF;
mBio. 11(3), 2020. Epub 2020/05/14. doi: 10.1128/mBio.00908-20. PMID: 32398315; PMC7218285. - Stress keratin 17 enhances papillomavirus infection-induced disease by downregulating T cell recruitment Wang W, Uberoi A, Spurgeon M, Gronski E, Majerciak V, Lobanov A, Hayes M, Loke A, Zheng ZM, Lambert PF;
PLoS Pathog. 16(1):e1008206, 2020. Epub 2020/01/23. doi: 10.1371/journal.ppat.1008206. PMID: 31968015; PMC6975545. - Mus musculus Papillomavirus 1: a New Frontier in Animal Models of Papillomavirus Pathogenesis Spurgeon ME, Lambert PF;
J Virol. 94(9), 2020. Epub 2020/02/14. doi: 10.1128/JVI.00002-20. PMID: 32051276; PMC7163119. - FGFR Inhibition Enhances Sensitivity to Radiation in Non-Small Cell Lung Cancer SenthilKumar G, Fisher MM, Skiba JH, Miller MC, Brennan SR, Kaushik S, Bradley ST, Longhurst CA, Buehler D, Nickel KP, Iyer G, Kimple RJ, Baschnagel AM;
Mol Cancer Ther. 19(6):1255-65, 2020. Epub 2020/05/07. doi: 10.1158/1535-7163.MCT-19-0931. PMID: 32371583; PMC7272291. - AXL Mediates Cetuximab and Radiation Resistance Through Tyrosine 821 and the c-ABL Kinase Pathway in Head and Neck Cancer McDaniel NK, Iida M, Nickel KP, Longhurst CA, Fischbach SR, Rodems TS, Kranjac CA, Bo AY, Luo Q, Gallagher MM, Welke NB, Mitchell KR, Schulz AE, Eckers JC, Hu R, Salgia R, Hong S, Bruce JY, Kimple RJ, Wheeler DL;
Clin Cancer Res. 26(16):4349-59, 2020. Epub 2020/05/23. doi: 10.1158/1078-0432.CCR-19-3142. PMID: 32439698; PMC7442604. - Priming and Propagating Anti-tumor Immunity: Focal Hypofractionated Radiation for in Situ Vaccination and Systemic Targeted Radionuclide Theranostics for Immunomodulation of Tumor Microenvironments Jagodinsky JC, Morris ZS;
Semin Radiat Oncol. 30(2):181-86, 2020. Epub 2020/05/10. doi: 10.1016/j.semradonc.2019.12.008. PMID: 32381297; PMC7286051. - The Promise of Combining Radiation Therapy With Immunotherapy Jagodinsky JC, Harari PM, Morris ZS;
Int J Radiat Oncol Biol Phys. 108(1):6-16, 2020. Epub 2020/04/27. doi: 10.1016/j.ijrobp.2020.04.023. PMID: 32335187; PMC7442714. - Targeting AKT/PKB to improve treatment outcomes for solid tumors Iida M, Harari PM, Wheeler DL, Toulany M;
Mutat Res. 819-820:111690, 2020. Epub 2020/03/03. doi: 10.1016/j.mrfmmm.2020.111690. PMID: 32120136; PMC7169978. - Life Beyond COVID: Pay Attention to Viruses Harari PM, Lambert PF;
Int J Radiat Oncol Biol Phys. 108(2):348-50, 2020. Epub 2020/09/06. doi: 10.1016/j.ijrobp.2020.07.001. PMID: 32890509; PMC7462873. - Interstitial diffuse optical probe with spectral fitting to measure dynamic tumor hypoxia Fru LC, Jacques SL, Nickel KP, Varghese T, Kissick MW, DeWerd LA, Kimple RJ;
Biomed Phys Eng Express. 6(1), 2020. Epub 2020/02/26. doi: 10.1088/2057-1976/ab6e16. PMID: 32095273; PMC7039661. - Fibroblast Growth Factor Receptors as Targets for Radiosensitization in Head and Neck Squamous Cell Carcinomas Fisher MM, SenthilKumar G, Hu R, Goldstein S, Ong IM, Miller MC, Brennan SR, Kaushik S, Abel L, Nickel KP, Iyer G, Harari PM, Kimple RJ, Baschnagel AM;
Int J Radiat Oncol Biol Phys. 107(4):793-803, 2020. Epub 2020/04/17. doi: 10.1016/j.ijrobp.2020.03.040. PMID: 32298810; PMC7321889. - Patient Derived Models to Study Head and Neck Cancer Radiation Response Cosper PF, Abel L, Lee YS, Paz C, Kaushik S, Nickel KP, Alexandridis R, Scott JG, Bruce JY, Kimple RJ;
Cancers (Basel). 12(2), 2020. Epub 2020/02/16. doi: 10.3390/cancers12020419. PMID: 32059418; PMC7072508. - Follow-Up and Management of Patients With Head and Neck Cancer During the 2019 Novel Coronavirus (SARS-CoV-2) Disease Pandemic Chua MLK, Ma DJ, Anderson CM, Karam SD, Margalit DN, Kimple RJ;
Adv Radiat Oncol. 5(4):631-36, 2020. Epub 2020/05/20. doi: 10.1016/j.adro.2020.04.031. PMID: 32426556; PMC7227497. - Clinical outcomes for larynx patients with cancer treated with refinement of high-dose radiation treatment volumes Burr AR, Harari PM, Haasl AM, Wieland AM, Bruce JY, Kimple RJ, Hartig GK, McCulloch TM, Witek ME;
Head Neck. 42(8):1874-81, 2020. Epub 2020/02/15. doi: 10.1002/hed.26098. PMID: 32057151; PMC7369226. - A Mouse Model of Oropharyngeal Papillomavirus-Induced Neoplasia Using Novel Tools for Infection and Nasal Anesthesia Bilger A, King RE, Schroeder JP, Piette JT, Hinshaw LA, Kurth AD, AlRamahi RW, Barthel MV, Ward-Shaw ET, Buehler D, Masters KS, Thibeault SL, Lambert PF;
Viruses. 12(4), 2020. Epub 2020/04/23. doi: 10.3390/v12040450. PMID: 32316091; PMC7232375. - Human Tumor-Lymphatic Microfluidic Model Reveals Differential Conditioning of Lymphatic Vessels by Breast Cancer Cells Ayuso JM, Gong MM, Skala MC, Harari PM, Beebe DJ;
Adv Healthc Mater. 9(3):e1900925, 2020. Epub 2020/01/03. doi: 10.1002/adhm.201900925. PMID: 31894641; PMC7004876. - Expression pattern of androgen receptor and AR-V7 in androgen-deprivation therapy-naive salivary duct carcinomas Yang RK, Zhao P, Lu C, Luo J, Hu R;
Hum Pathol. 84:173-82, 2019. Epub 2018/09/30. doi: 10.1016/j.humpath.2018.09.009. PMID: 30267779; PMC7098468. - High-throughput quantitative detection of basal autophagy and autophagic flux using image cytometry SenthilKumar G, Skiba JH, Kimple RJ;
Biotechniques. 67(2):70-73, 2019. Epub 2019/06/27. doi: 10.2144/btn-2019-0044. PMID: 31238709; PMC7141596. - Development of an In Situ Cancer Vaccine via Combinational Radiation and Bacterial-Membrane-Coated Nanoparticles Patel RB, Ye M, Carlson PM, Jaquish A, Zangl L, Ma B, Wang Y, Arthur I, Xie R, Brown RJ, Wang X, Sriramaneni R, Kim K, Gong S, Morris ZS;
Adv Mater. 31(43):e1902626, 2019. Epub 2019/09/17. doi: 10.1002/adma.201902626. PMID: 31523868; PMC6810793. - Dendrimer-Based Platform for Effective Capture of Tumor Cells after TGFbeta1-Induced Epithelial-Mesenchymal Transition Myung JH, Cha A, Tam KA, Poellmann M, Borgeat A, Sharifi R, Molokie RE, Votta-Velis G, Hong S;
Anal Chem. 91(13):8374-82, 2019. Epub 2019/06/30. doi: 10.1021/acs.analchem.9b01181. PMID: 31247718; PMC7068806. - A Phase Ib Study of Axitinib in Combination with Crizotinib in Patients with Metastatic Renal Cell Cancer or Other Advanced Solid Tumors Michaelson MD, Gupta S, Agarwal N, Szmulewitz R, Powles T, Pili R, Bruce JY, Vaishampayan U, Larkin J, Rosbrook B, Wang E, Murphy D, Wang P, Lechuga MJ, Valota O, Shepard DR;
Oncologist. 24(9):1151-e817, 2019. Epub 2019/06/07. doi: 10.1634/theoncologist.2018-0749. PMID: 31171735; PMC6738313. - TAM Family proteins and therapy resistance McDaniel NK, Fischbach SR, Ondrack O, Welke N, Iida M, Wheeler DL;
Improving the therapeutic ratio in head and neck cancer. 1st ed: Academic Press; 2019. p. 159-92. - Preclinical Pharmacokinetics and Dosimetry Studies of (124)I/(131)I-CLR1404 for Treatment of Pediatric Solid Tumors in Murine Xenograft Models Marsh IR, Grudzinski J, Baiu DC, Besemer A, Hernandez R, Jeffery JJ, Weichert JP, Otto M, Bednarz BP;
J Nucl Med. 60(10):1414-20, 2019. Epub 2019/03/31. doi: 10.2967/jnumed.118.225409. PMID: 30926646; PMC6785791. - Age-related alterations in swallowing biomechanics Kletzien H, Cullins MJ, Connor NP;
Exp Gerontol. 118:45-50, 2019. Epub 2019/01/12. doi: 10.1016/j.exger.2019.01.006. PMID: 30633957; PMC6430567. - SLP-Perceived Technical and Patient-Centered Factors Associated with Pharyngeal High-Resolution Manometry Jones CA, Rogus-Pulia NM, Forgues AL, Orne J, Macdonald CL, Connor NP, McCulloch TM;
Dysphagia. 34(2):170-78, 2019. Epub 2018/11/02. doi: 10.1007/s00455-018-9954-z. PMID: 30382385; PMC6422684. - Correlates of Early Pharyngeal High-Resolution Manometry Adoption in Expert Speech-Language Pathologists Jones CA, Forgues AL, Rogus-Pulia NM, Orne J, Macdonald CL, Connor NP, McCulloch TM;
Dysphagia. 34(3):325-32, 2019. Epub 2018/09/21. doi: 10.1007/s00455-018-9941-4. PMID: 30232550; PMC6424656. - Human organotypic lymphatic vessel model elucidates microenvironment-dependent signaling and barrier function Gong MM, Lugo-Cintron KM, White BR, Kerr SC, Harari PM, Beebe DJ;
Biomaterials. 214:119225, 2019. Epub 2019/06/04. doi: 10.1016/j.biomaterials.2019.119225. PMID: 31154151; PMC7032699. - The Specificity of EGF-Stimulated IQGAP1 Scaffold Towards the PI3K-Akt Pathway is Defined by the IQ3 motif Chen M, Choi S, Jung O, Wen T, Baum C, Thapa N, Lambert PF, Rapraeger AC, Anderson RA;
Sci Rep. 9(1):9126, 2019. Epub 2019/06/27. doi: 10.1038/s41598-019-45671-5. PMID: 31235839; PMC6591252. - Reducing radiotherapy target volume expansion for patients with HPV-associated oropharyngeal cancer Burr AR, Harari PM, Ko HC, Bruce JY, Kimple RJ, Witek ME;
Oral Oncol. 92:52-56, 2019. Epub 2019/04/24. doi: 10.1016/j.oraloncology.2019.03.013. PMID: 31010623; PMC7062456. - Pretreatment CLR 124 Positron Emission Tomography Accurately Predicts CLR 131 Three-Dimensional Dosimetry in a Triple-Negative Breast Cancer Patient Besemer AE, Grudzinski JJ, Weichert JP, Hall LT, Bednarz BP;
Cancer Biother Radiopharm. 34(1):13-23, 2019. Epub 2018/10/24. doi: 10.1089/cbr.2018.2568. PMID: 30351218; PMC6383576. - Effects of culture method on response to EGFR therapy in head and neck squamous cell carcinoma cells Ayuso JM, Vitek R, Swick AD, Skala MC, Wisinski KB, Kimple RJ, Lambert PF, Beebe DJ;
Sci Rep. 9(1):12480, 2019. Epub 2019/08/30. doi: 10.1038/s41598-019-48764-3. PMID: 31462653; PMC6713778. - De-Escalation Strategies in HPV-Associated Oropharynx Cancer-Are we Putting the Cart Before the Horse? Int J Radiat Oncol Biol Phys Anderson CM, Kimple RJ, Lin A, Karam SD, Margalit DN, Chua MLK;
104(4):705-09, 2019. Epub 2019/06/18. doi: 10.1016/j.ijrobp.2019.02.054. PMID: 31204653; PMC7194352. - Is HPV-Associated Oropharyngeal Cancer Becoming More Common in Older Patients? Laryngoscope Investig Otolaryngol Thompson JD, Harari PM, Hartig GK;
3(6):446-49, 2018. Epub 2019/01/02. doi: 10.1002/lio2.181. PMID: 30599028; PMC6302704. - Pharmacodynamic study using FLT PET/CT in advanced solid malignancies treated with a sequential combination of X-82 and docetaxel Scarpelli M, Rampurwala M, Eickhoff J, Carmichael L, Heideman J, Binger K, Kolesar J, Perlman S, Harrow K, Dukart G, Liang C, Jeraj R, Liu G, Bruce JY;
Cancer Chemother Pharmacol. 82(2):211-19, 2018. Epub 2018/05/29. doi: 10.1007/s00280-018-3599-3. PMID: 29802443; PMC7205037. - A Pilot Study of Perceived Mouth Dryness, Perceived Swallowing Effort, and Saliva Substitute Effects in Healthy Adults Across the Age Range Rogus-Pulia NM, Gangnon R, Kind A, Connor NP, Asthana S;
Dysphagia. 33(2):200-05, 2018. Epub 2017/09/08. doi: 10.1007/s00455-017-9846-7. PMID: 28879557; PMC7061950. - Anti-Trop2 blockade enhances the therapeutic efficacy of ErbB3 inhibition in head and neck squamous cell carcinoma Redlich N, Robinson AM, Nickel KP, Stein AP, Wheeler DL, Adkins DR, Uppaluri R, Kimple RJ, Van Tine BA, Michel LS;
Cell Death Dis. 9(1):5, 2018. Epub 2018/01/07. doi: 10.1038/s41419-017-0029-0. PMID: 29305574; PMC5849045. - Overcoming Resistance to Cetuximab with Honokiol, A Small-Molecule Polyphenol Pearson HE, Iida M, Orbuch RA, McDaniel NK, Nickel KP, Kimple RJ, Arbiser JL, Wheeler DL;
Mol Cancer Ther. 17(1):204-14, 2018. Epub 2017/10/22. doi: 10.1158/1535-7163.MCT-17-0384. PMID: 29054984; PMC5752575. - Loss of Function of Canonical Notch Signaling Drives Head and Neck Carcinogenesis Nyman PE, Buehler D, Lambert PF;
Clin Cancer Res. 24(24):6308-18, 2018. Epub 2018/08/09. doi: 10.1158/1078-0432.CCR-17-3535. PMID: 30087145; PMC6295262. - Multivalent Binding and Biomimetic Cell Rolling Improves the Sensitivity and Specificity of Circulating Tumor Cell Capture Myung JH, Eblan MJ, Caster JM, Park SJ, Poellmann MJ, Wang K, Tam KA, Miller SM, Shen C, Chen RC, Zhang T, Tepper JE, Chera BS, Wang AZ, Hong S;
Clin Cancer Res. 24(11):2539-47, 2018. Epub 2018/03/17. doi: 10.1158/1078-0432.CCR-17-3078. PMID: 29545463; PMC5984698. - Tumor-Specific Inhibition of In Situ Vaccination by Distant Untreated Tumor Sites Morris ZS, Guy EI, Werner LR, Carlson PM, Heinze CM, Kler JS, Busche SM, Jaquish AA, Sriramaneni RN, Carmichael LL, Loibner H, Gillies SD, Korman AJ, Erbe AK, Hank JA, Rakhmilevich AL, Harari PM, Sondel PM;
Cancer Immunol Res. 6(7):825-34, 2018. Epub 2018/05/12. doi: 10.1158/2326-6066.CIR-17-0353. PMID: 29748391; PMC6030484. - MERTK Mediates Intrinsic and Adaptive Resistance to AXL-targeting Agents McDaniel NK, Cummings CT, Iida M, Hulse J, Pearson HE, Vasileiadi E, Parker RE, Orbuch RA, Ondracek OJ, Welke NB, Kang GH, Davies KD, Wang X, Frye SV, Earp HS, Harari PM, Kimple RJ, DeRyckere D, Graham DK, Wheeler DL;
Mol Cancer Ther. 17(11):2297-308, 2018. Epub 2018/08/11. doi: 10.1158/1535-7163.MCT-17-1239. PMID: 30093568; PMC6215511. - Modeling Cell and Tumor-Metastasis Dosimetry with the Particle and Heavy Ion Transport Code System (PHITS) Software for Targeted Alpha-Particle Radionuclide Therapy Lee D, Li M, Bednarz B, Schultz MK;
Radiat Res. 190(3):236-47, 2018. Epub 2018/06/27. doi: 10.1667/RR15081.1. PMID: 29944461; PMC6512332. - Patient-Reported Dysphagia After Thyroidectomy: A Qualitative Study Krekeler BN, Wendt E, Macdonald C, Orne J, Francis DO, Sippel R, Connor NP;
JAMA Otolaryngol Head Neck Surg. 144(4):342-48, 2018. Epub 2018/03/10. doi: 10.1001/jamaoto.2017.3378. PMID: 29522149; PMC5876907. - Tongue exercise and ageing effects on morphological and biochemical properties of the posterior digastric and temporalis muscles in a Fischer 344 Brown Norway rat model Krekeler BN, Leverson G, Connor NP;
Arch Oral Biol. 89:37-43, 2018. Epub 2018/02/14. doi: 10.1016/j.archoralbio.2018.02.002. PMID: 29438907; PMC5869113. - Patient Adherence to Dysphagia Recommendations: A Systematic Review Krekeler BN, Broadfoot CK, Johnson S, Connor NP, Rogus-Pulia N;
Dysphagia. 33(2):173-84, 2018. Epub 2017/10/02. doi: 10.1007/s00455-017-9852-9. PMID: 28965240; PMC5866734. - Effect of neuromuscular electrical stimulation frequency on muscles of the tongue Kletzien H, Russell JA, Leverson G, Connor NP;
Muscle Nerve. 58(3):441-48, 2018. Epub 2018/05/26. doi: 10.1002/mus.26173. PMID: 29797723; PMC6160334. - Comparison Between Patient-Perceived Voice Changes and Quantitative Voice Measures in the First Postoperative Year After Thyroidectomy: A Secondary Analysis of a Randomized Clinical Trial Kletzien H, Macdonald CL, Orne J, Francis DO, Leverson G, Wendt E, Sippel RS, Connor NP;
JAMA Otolaryngol Head Neck Surg. 144(11):995-1003, 2018. Epub 2018/05/02. doi: 10.1001/jamaoto.2018.0309. PMID: 29710208; PMC6193861. - Age-related effect of cell death on fiber morphology and number in tongue muscle Kletzien H, Hare AJ, Leverson G, Connor NP;
Muscle Nerve. 57(1):E29-E37, 2018. Epub 2017/04/26. doi: 10.1002/mus.25671. PMID: 28440544; PMC5656552. - Peptide-nanoparticle conjugates: a next generation of diagnostic and therapeutic platforms? Nano Converg Jeong WJ, Bu J, Kubiatowicz LJ, Chen SS, Kim Y, Hong S;
5(1):38, 2018. Epub 2018/12/13. doi: 10.1186/s40580-018-0170-1. PMID: 30539365; PMC6289934. - Genomics Reloaded: Rise of the Expression Profiles Gan GN, Kimple RJ;
Int J Radiat Oncol Biol Phys. 101(1):1-3, 2018. Epub 2018/04/06. doi: 10.1016/j.ijrobp.2017.10.023. PMID: 29619961; PMC6821516. - Enhanced Radiosensitivity in Solid Tumors using a Tumor-selective Alkyl Phospholipid Ether Analog Elsaid MY, Shahi A, Wang AR, Baiu DC, Li C, Werner LR, Singhal S, Hall LT, Weichert JP, Armstrong EA, Bednarz BP, Harari PM, Iyer G, Otto M;
Mol Cancer Ther. 17(11):2320-28, 2018. Epub 2018/08/16. doi: 10.1158/1535-7163.MCT-17-0897. PMID: 30108133; PMC6215514. - Impact of HPV Status on the Prognostic Potential of the AJCC Staging System for Larynx Cancer Davidson SM, Ko HC, Harari PM, Wieland AM, Chen S, Baschnagel AM, Kimple RJ, Witek ME;
Otolaryngol Head Neck Surg. 159(3):456-65, 2018. Epub 2018/04/04. doi: 10.1177/0194599818766035. PMID: 29611770; PMC7141595. - Differential impact of tongue exercise on intrinsic lingual muscles Cullins MJ, Krekeler BN, Connor NP;
Laryngoscope. 128(10):2245-51, 2018. Epub 2017/12/16. doi: 10.1002/lary.27044. PMID: 29243257; PMC6003827. - HPV impacts survival of stage IVC non-oropharyngeal HNSCC cancer patients Burr AR, Harari PM, Ko HC, Chen S, Yu M, Baschnagel AM, Kimple RJ, Witek ME;
Otorhinolaryngol Head Neck Surg. 3(1), 2018. Epub 2018/10/03. doi: 10.15761/OHNS.1000160. PMID: 30271885; PMC6157736. - Results From 10 Years of a Free Oral Cancer Screening Clinic at a Major Academic Health Center Blitzer GC, Rosenberg SA, Anderson BM, McCulloch TM, Wieland AM, Hartig GK, Bruce JY, Witek ME, Kimple RJ, Harari PM;
Int J Radiat Oncol Biol Phys. 102(1):146-48, 2018. Epub 2018/07/08. doi: 10.1016/j.ijrobp.2018.05.007. PMID: 29980415; PMC6089656. - Development and Validation of RAPID: A Patient-Specific Monte Carlo Three-Dimensional Internal Dosimetry Platform Besemer AE, Yang YM, Grudzinski JJ, Hall LT, Bednarz BP;
Cancer Biother Radiopharm. 33(4):155-65, 2018. Epub 2018/04/26. doi: 10.1089/cbr.2018.2451. PMID: 29694246; PMC5963670. - Murine-specific Internal Dosimetry for Preclinical Investigations of Imaging and Therapeutic Agents Bednarz B, Grudzinski J, Marsh I, Besemer A, Baiu D, Weichert J, Otto M;
Health Phys. 114(4):450-59, 2018. Epub 2018/02/27. doi: 10.1097/HP.0000000000000789. PMID: 29481536; PMC5831541. - Outcomes for patients with head and neck squamous cell carcinoma presenting with N3 nodal disease Witek ME, Wieland AM, Chen S, Kennedy TA, Hullett CR, Liang E, Hartig GK, Kimple RJ, Harari PM;
Cancers Head Neck. 2, 2017. Epub 2017/01/01. doi: 10.1186/s41199-017-0027-z. PMID: 29527332; PMC5844268. - Transcriptional-mediated effects of radiation on the expression of immune susceptibility markers in melanoma Werner LR, Kler JS, Gressett MM, Riegert M, Werner LK, Heinze CM, Kern JG, Abbariki M, Erbe AK, Patel RB, Sriramaneni RN, Harari PM, Morris ZS;
Radiother Oncol. 124(3):418-26, 2017. Epub 2017/09/13. doi: 10.1016/j.radonc.2017.08.016. PMID: 28893414; PMC5626442. - Cotargeting mTORC and EGFR Signaling as a Therapeutic Strategy in HNSCC Swick AD, Prabakaran PJ, Miller MC, Javaid AM, Fisher MM, Sampene E, Ong IM, Hu R, Iida M, Nickel KP, Bruce JY, Wheeler DL, Kimple RJ;
Mol Cancer Ther. 16(7):1257-68, 2017. Epub 2017/04/28. doi: 10.1158/1535-7163.MCT-17-0115. PMID: 28446642; PMC5505754. - Papillary Thyroid Cancer: The Good and Bad of the “Good Cancer” Randle RW, Bushman NM, Orne J, Balentine CJ, Wendt E, Saucke M, Pitt SC, Macdonald CL, Connor NP, Sippel RS;
Thyroid. 27(7):902-07, 2017. Epub 2017/05/17. doi: 10.1089/thy.2016.0632. PMID: 28510505; PMC5561445. - Radiosensitization of Adenoid Cystic Carcinoma with MDM2 Inhibition Prabakaran PJ, Javaid AM, Swick AD, Werner LR, Nickel KP, Sampene E, Hu R, Ong IM, Bruce JY, Hartig GK, Wieland AM, Canon J, Harari PM, Kimple RJ;
Clin Cancer Res. 23(20):6044-53, 2017. Epub 2017/07/01. doi: 10.1158/1078-0432.CCR-17-0969. PMID: 28659312; PMC5641244. - Age-related changes in mastication are not improved by tongue exercise in a rat model Krekeler BN, Connor NP;
Laryngoscope. 127(1):E29-E34, 2017. Epub 2016/06/05. doi: 10.1002/lary.26045. PMID: 27260802; PMC5136355. - Prognostic implications of human papillomavirus status for patients with non-oropharyngeal head and neck squamous cell carcinomas Ko HC, Harari PM, Sacotte RM, Chen S, Wieland AM, Yu M, Baschnagel AM, Bruce JY, Kimple RJ, Witek ME;
J Cancer Res Clin Oncol. 143(11):2341-50, 2017. Epub 2017/07/29. doi: 10.1007/s00432-017-2481-8. PMID: 28752235; PMC7069668. - Survival Outcomes for Patients With T3N0M0 Squamous Cell Carcinoma of the Glottic Larynx Ko HC, Harari PM, Chen S, Wieland AM, Yu M, Baschnagel AM, Kimple RJ, Witek ME;
JAMA Otolaryngol Head Neck Surg. 143(11):1126-33, 2017. Epub 2017/10/20. doi: 10.1001/jamaoto.2017.1756. PMID: 29049434; PMC5710357. - Clinical outcomes for patients presenting with N3 head and neck squamous cell carcinoma: Analysis of the National Cancer Database Ko HC, Chen S, Wieland AM, Yu M, Baschnagel AM, Hartig GK, Harari PM, Witek ME;
Head Neck. 39(11):2159-70, 2017. Epub 2017/07/25. doi: 10.1002/hed.24881. PMID: 28737019; PMC5647211. - Alterations of intrinsic tongue muscle properties with aging Cullins MJ, Connor NP;
Muscle Nerve. 56(6):E119-E25, 2017. Epub 2017/02/10. doi: 10.1002/mus.25605. PMID: 28181263; PMC5550369. - The receptor tyrosine kinase AXL mediates nuclear translocation of the epidermal growth factor receptor Brand TM, Iida M, Corrigan KL, Braverman CM, Coan JP, Flanigan BG, Stein AP, Salgia R, Rolff J, Kimple RJ, Wheeler DL;
Sci Signal. 10(460), 2017. Epub 2017/01/05. doi: 10.1126/scisignal.aag1064. PMID: 28049763; PMC7094775.
Photo Album
2019


2017


2016











2014





2013
2012


2008

2006


Sergio Farewell dinner
2004

2002

-
Safety and toxicity of Iopofosine I 131 (CLR 131) with external beam radiation therapy in recurrent or metastatic head and neck cancer: results of a phase 1 single-centre, open-label, single-arm, dose escalation and dose expansion study EBioMedicine
Bruce JY, Burr A, Kimple RJ, Adam DP, Yu M, Piaskowski SM, Glazer TA, Hill P, Hartig GK, McCulloch TM, Wieland AM, Trask D, Oliver K, Longcor J, Rogus-Pulia N, Cho SY, Bednarz B, Harari PM
2025 Jan;111:105496. doi: 10.1016/j.ebiom.2024.105496. Epub 2024 Dec 12.
-
More
BACKGROUND: Re-irradiation of recurrent head and neck cancer (HNC) is often limited by tumour adherence to critical structures and/or radiation tolerance of critical normal tissues. Iopofosine I 131 (CLR 131) is a targeted small molecular phospholipid ether (PLE) drug conjugate that delivers iodine-131 selectively to tumour cells. We conducted a phase 1, single-centre, open-label study to determine whether CLR 131 given with reduced dose of external beam radiation therapy (EBRT) would be tolerable and feasible.
METHODS: All participants received previous curative intent treatment with radiotherapy as primary or adjuvant treatment. Eligible participants demonstrated uptake of CLR 131 as indicated via single photon emission CT/CT (SPECT/CT) imaging following CLR 131 test dose. Participants received two therapeutic doses of CLR 131 (days 1 and 8) with SPECT/CT imaging performed to quantitate the biodistribution of CLR 131. Participants subsequently received EBRT to achieve the designated radiation dose (60-70 Gy). The primary endpoint was safety. This trial was registered with ClinicalTrials.gov, NCT04105543, and enrolment and follow-up are complete.
FINDINGS: Twelve participants completed treatment with CLR 131 and EBRT. Eight participants experienced grade 4 non-DLT haematologic toxicities (2 anaemia, 8 leukopenia, 5 thrombocytopenia) at least probably attributed to CLR 131, consistent with the expected toxicity profile. Haematologic toxicities occurred during weeks 6-8 from the first dose of CLR 131 and resolved within three weeks without sequelae. There were no treatment-related grade 3-4 non-haematologic toxicities.
INTERPRETATION: CLR 131 in combination with EBRT did not confer any safety concerns, and was tolerable in participants with recurrent/metastatic HNC. Myelosuppression was consistent with the known toxicity profile of CLR 131.
FUNDING: National Institutes of HealthP50 DE026787, National Cancer InstituteP30 CA014520, National Institutes of Health1UL1TR002373, Cellectar, NCT04105543.
PMID:39671752 | PMC:PMC11700259 | DOI:10.1016/j.ebiom.2024.105496
View details for PubMedID 39671752
-
More
-
From Bench to Bedside: A Team's Approach to Multidisciplinary Strategies to Combat Therapeutic Resistance in Head and Neck Squamous Cell Carcinoma Journal of clinical medicine
Crossman BE, Harmon RL, Kostecki KL, McDaniel NK, Iida M, Corday LW, Glitchev CE, Crow MT, Harris MA, Lin CY, Adams JM, Longhurst CA, Nickel KP, Ong IM, Alexandridis RA, Yu M, Yang DT, Hu R, Morris ZS, Hartig GK, Glazer TA, Ramisetty S, Kulkarni P, Salgia R, Kimple RJ, Bruce JY, Harari PM, Wheeler DL
2024 Oct 10;13(20):6036. doi: 10.3390/jcm13206036.
-
More
Head and neck squamous cell carcinoma (HNSCC) is diagnosed in more than 71,000 patients each year in the United States, with nearly 16,000 associated deaths. One significant hurdle in the treatment of HNSCC is acquired and intrinsic resistance to existing therapeutic agents. Over the past several decades, the University of Wisconsin has formed a multidisciplinary team to move basic scientific discovery along the translational spectrum to impact the lives of HNSCC patients. In this review, we outline key discoveries made throughout the years at the University of Wisconsin to deepen our understanding of therapeutic resistance in HNSCC and how a strong, interdisciplinary team can make significant advances toward improving the lives of these patients by combatting resistance to established therapeutic modalities. We are profoundly grateful to the many scientific teams worldwide whose groundbreaking discoveries, alongside evolving clinical paradigms in head and neck oncology, have been instrumental in making our work possible.
PMID:39457986 | PMC:PMC11508784 | DOI:10.3390/jcm13206036
View details for PubMedID 39457986
-
More
-
Uncommon and Challenging Phenotypes of High-Risk Human Papillomavirus-Associated Head and Neck Carcinomas Revealed by High-Throughput Studies Head and neck pathology
Tannenbaum AP, Lozar T, Lu C, Schumacher M, Golfinos A, Dinh HQ, Taylor N, Kimple RJ, Yang D, Harari PM, Lambert PF, Lloyd RV, Hu R
2024 Oct 22;18(1):112. doi: 10.1007/s12105-024-01707-5.
-
More
BACKGROUND: HPV- associated squamous cell carcinoma (SCC) is uncommon in non-oropharynx sites and not well characterized. This study aims to investigate uncommon phenotypes of HPV-associated head and neck carcinoma, the prevalence and morphologic spectrum of HPV-associated SCC in the oral cavity, larynx and hypopharynx.
METHOD: P16 immunostaining and HPV E6/7 in situ hybridization (ISH) were performed on tissue microarrays comprised of SCCs from different anatomic sites: oropharynx (n = 270), hypopharynx (n = 52), oral cavity (n = 95) and larynx (n = 123). Tumors were classified as HPV-associated based on a positive E6/7 ISH testing. RNA sequencing was performed on several selected cases.
RESULT: 66% oropharynx SCCs (OPSCCs) were HPV-associated; all were p16/HPV testing concordant except one which was p16 negative. The p16-/HPV + OPSCC resembled similar gene expression signature with p16+/HPV + OPSCCs by transcriptome analysis. 6/95 (6%) oral cavity SCCs were HPV-associated, all from male patients and 5/6 (83%) arose from the floor of mouth. Morphologically, 3/6 (50%) showed keratinizing SCC and 5/6 (83%) demonstrated HPV-associated squamous dysplasia in adjacent mucosa. 1/123 (less than 1%) larynx SCCs and 0/52 hypopharynx SCCs were HPV-associated.
CONCLUSION: Although uncommon, p16 negative HPV-associated OPSCC can occur, emphasizing the importance of judicious HPV testing. The morphology of HPV-associated oral cavity SCCs may deviate from prototypic nonkeratinizing SCC, making them difficult to recognize. Presence of HPV-associated squamous dysplasia could serve as a morphologic clue.
PMID:39436498 | PMC:PMC11496466 | DOI:10.1007/s12105-024-01707-5
View details for PubMedID 39436498
-
More
-
Chromosomal instability increases radiation sensitivity bioRxiv : the preprint server for biology
Cosper PF, Paracha M, Jones KM, Hrycyniak L, Henderson L, Bryan A, Eyzaguirre D, McCunn E, Boulanger E, Wan J, Nickel KP, Horner V, Hu R, Harari PM, Kimple RJ, Weaver BA
2024 Sep 19:2024.09.13.612942. doi: 10.1101/2024.09.13.612942.
-
More
Continuous chromosome missegregation over successive mitotic divisions, known as chromosomal instability (CIN), is common in cancer. Increasing CIN above a maximally tolerated threshold leads to cell death due to loss of essential chromosomes. Here, we show in two tissue contexts that otherwise isogenic cancer cells with higher levels of CIN are more sensitive to ionizing radiation, which itself induces CIN. CIN also sensitizes HPV-positive and HPV-negative head and neck cancer patient derived xenograft (PDX) tumors to radiation. Moreover, laryngeal cancers with higher CIN prior to treatment show improved response to radiation therapy. In addition, we reveal a novel mechanism of radiosensitization by docetaxel, a microtubule stabilizing drug commonly used in combination with radiation. Docetaxel causes cell death by inducing CIN due to abnormal multipolar spindles rather than causing mitotic arrest, as previously assumed. Docetaxel-induced CIN, rather than mitotic arrest, is responsible for the enhanced radiation sensitivity observed in vitro and in vivo, challenging the mechanistic dogma of the last 40 years. These results implicate CIN as a potential biomarker and inducer of radiation response, which could provide valuable cancer therapeutic opportunities.
STATEMENT OF SIGNIFICANCE: Cancer cells and laryngeal tumors with higher chromosome missegregation rates are more sensitive to radiation therapy, supporting chromosomal instability as a promising biomarker of radiation response.
PMID:39345631 | PMC:PMC11429890 | DOI:10.1101/2024.09.13.612942
View details for PubMedID 39345631
-
More
-
Intratumoral radiation dose heterogeneity augments antitumor immunity in mice and primes responses to checkpoint blockade Science translational medicine
Jagodinsky JC, Vera JM, Jin WJ, Shea AG, Clark PA, Sriramaneni RN, Havighurst TC, Chakravarthy I, Allawi RH, Kim K, Harari PM, Sondel PM, Newton MA, Crittenden MR, Gough MJ, Miller JR, Ong IM, Morris ZS
2024 Sep 18;16(765):eadk0642. doi: 10.1126/scitranslmed.adk0642. Epub 2024 Sep 18.
-
More
Radiation therapy (RT) activates multiple immunologic effects in the tumor microenvironment (TME), with diverse dose-response relationships observed. We hypothesized that, in contrast with homogeneous RT, a heterogeneous RT dose would simultaneously optimize activation of multiple immunogenic effects in a single TME, resulting in a more effective antitumor immune response. Using high-dose-rate brachytherapy, we treated mice bearing syngeneic tumors with a single fraction of heterogeneous RT at a dose ranging from 2 to 30 gray. When combined with dual immune checkpoint inhibition in murine models, heterogeneous RT generated more potent antitumor responses in distant, nonirradiated tumors compared with any homogeneous dose. The antitumor effect after heterogeneous RT required CD4 and CD8 T cells and low-dose RT to a portion of the tumor. At the 3-day post-RT time point, dose heterogeneity imprinted the targeted TME with spatial differences in immune-related gene expression, antigen presentation, and susceptibility of tumor cells to immune-mediated destruction. At a later 10-day post-RT time point, high-, moderate-, or low-RT-dose regions demonstrated distinct infiltrating immune cell populations. This was associated with an increase in the expression of effector-associated cytokines in circulating CD8 T cells. Consistent with enhanced adaptive immune priming, heterogeneous RT promoted clonal expansion of effector CD8 T cells. These findings illuminate the breadth of dose-dependent effects of RT on the TME and the capacity of heterogeneous RT to promote antitumor immunity when combined with immune checkpoint inhibitors.
PMID:39292804 | PMC:PMC11522033 | DOI:10.1126/scitranslmed.adk0642
View details for PubMedID 39292804
-
More
-
Morphologic Spectrum of HPV-associated Sinonasal Carcinomas Head and neck pathology
Abi-Saab T, Lozar T, Chen Y, Tannenbaum AP, Geye H, Yu M, Weisman P, Harari PM, Kimple RJ, Lambert PF, Lloyd RV, Hu R
2024 Aug 5;18(1):67. doi: 10.1007/s12105-024-01670-1.
-
More
BACKGROUND: High-risk human papillomavirus (HR-HPV) infection has been increasingly recognized as a risk factor for sinonasal tract carcinomas. However the prevalence and prognostic significance of HPV-associated sinonasal carcinomas is not well known due to limited studies and inconsistency in HPV testing modalities in literatures. Morphologically, HPV-associated sinonasal carcinomas encompass a diverse group of tumors. HPV-associated sinonasal adenocarcinoma has not been reported. The purpose of this study was to determine the prevalence, morphologic spectrum and prognostic implication of HPV-associated sinonasal carcinomas.
METHODS: This cohort included 153 sinonasal carcinomas. Tissue microarrays were constructed. P16 immunohistochemistry and HR-HPV E6/7 in-situ Hybridization (ISH) were performed. Carcinomas were deemed HPV-associated based on a positive ISH testing. Clinicopathologic data was collected.
RESULTS: 28/153 (18%) sinonasal carcinomas were HPV-associated. HPV-associated carcinomas consisted of 26 (93%) squamous cell carcinomas and variants, 1 (3.5%) HPV-related multiphenotypic sinonasal carcinoma and 1 (3.5%) adenocarcinoma. The HPV-associated adenocarcinoma closely resembled HPV-associated endocervical adenocarcinoma morphologically. HPV-associated carcinomas occurred in 8 (29%) women and 20 (71%) men with a median age of 66 years old. HPV-associated carcinomas were predominantly located at nasal cavity. A trend toward improved overall survival and progression free survival in HPV-associated carcinomas patients was observed, yet without statistical significance.
CONCLUSION: Our study identifies a novel HPV-associated sinonasal adenocarcinoma subtype, highlights the broad morphologic spectrum of HPV-associated sinonasal carcinomas, and supports routine p16 testing during pathology practice regardless of tumor subtype followed by a confirmatory HR-HPV testing. This practice is critical for studying the clinical behavior of HPV-associated sinonasal carcinomas.
PMID:39101976 | PMC:PMC11300749 | DOI:10.1007/s12105-024-01670-1
View details for PubMedID 39101976
-
More
-
Radiation Therapy for HPV-Positive Oropharyngeal Squamous Cell Carcinoma: An ASTRO Clinical Practice Guideline Practical radiation oncology
Margalit DN, Anker CJ, Aristophanous M, Awan M, Bajaj GK, Bradfield L, Califano J, Caudell JJ, Chapman CH, Garden AS, Harari PM, Helms A, Lin A, Maghami E, Mehra R, Parker L, Shnayder Y, Spencer S, Swiecicki PL, Tsai JC, Sher DJ
2024 Sep-Oct;14(5):398-425. doi: 10.1016/j.prro.2024.05.007. Epub 2024 Jun 18.
-
More
PURPOSE: Human Papilloma Virus (HPV)-associated oropharyngeal squamous cell carcinoma (OPSCC) is a distinct disease from other head and neck tumors. This guideline provides evidence-based recommendations on the critical decisions in its curative treatment, including both definitive and postoperative radiation therapy (RT) management.
METHODS: ASTRO convened a task force to address 5 key questions on the use of RT for management of HPV-associated OPSCC. These questions included indications for definitive and postoperative RT and chemoradiation; dose-fractionation regimens and treatment volumes; preferred RT techniques and normal tissue considerations; and posttreatment management decisions. The task force did not address indications for primary surgery versus RT. Recommendations were based on a systematic literature review and created using a predefined consensus-building methodology and system for grading evidence quality and recommendation strength.
RESULTS: Concurrent cisplatin is recommended for patients receiving definitive RT with T3-4 disease and/or 1 node >3 cm, or multiple nodes. For similar patients who are ineligible for cisplatin, concurrent cetuximab, carboplatin/5-fluorouracil, or taxane-based systemic therapy are conditionally recommended. In the postoperative setting, RT with concurrent cisplatin (either schedule) is recommended for positive surgical margins or extranodal extension. Postoperative RT alone is recommended for pT3-4 disease, >2 nodes, or a single node >3 cm. Observation is conditionally recommended for pT1-2 disease and a single node ≤3 cm without other risk factors. For patients treated with definitive RT with concurrent systemic therapy, 7000 cGy in 33 to 35 fractions is recommended, and for patients receiving postoperative RT without positive surgical margins and extranodal extension, 5600 to 6000 cGy is recommended. For all patients receiving RT, intensity modulated RT over 3-dimensional techniques with reduction in dose to critical organs at risk (including salivary and swallowing structures) is recommended. Reassessment with positron emission tomography-computed tomography is recommended approximately 3 months after definitive RT/chemoradiation, and neck dissection is recommended for convincing evidence of residual disease; for equivocal positron emission tomography-computed tomography findings, either neck dissection or repeat imaging is recommended.
CONCLUSIONS: The role and practice of RT continues to evolve for HPV-associated OPSCC, and these guidelines inform best clinical practice based on the available evidence.
PMID:39078350 | DOI:10.1016/j.prro.2024.05.007
View details for PubMedID 39078350
-
More
-
A platform-independent AI tumor lineage and site (ATLAS) classifier Communications biology
Rydzewski NR, Shi Y, Li C, Chrostek MR, Bakhtiar H, Helzer KT, Bootsma ML, Berg TJ, Harari PM, Floberg JM, Blitzer GC, Kosoff D, Taylor AK, Sharifi MN, Yu M, Lang JM, Patel KR, Citrin DE, Sundling KE, Zhao SG
2024 Mar 13;7(1):314. doi: 10.1038/s42003-024-05981-5.
-
More
Histopathologic diagnosis and classification of cancer plays a critical role in guiding treatment. Advances in next-generation sequencing have ushered in new complementary molecular frameworks. However, existing approaches do not independently assess both site-of-origin (e.g. prostate) and lineage (e.g. adenocarcinoma) and have minimal validation in metastatic disease, where classification is more difficult. Utilizing gradient-boosted machine learning, we developed ATLAS, a pair of separate AI Tumor Lineage and Site-of-origin models from RNA expression data on 8249 tumor samples. We assessed performance independently in 10,376 total tumor samples, including 1490 metastatic samples, achieving an accuracy of 91.4% for cancer site-of-origin and 97.1% for cancer lineage. High confidence predictions (encompassing the majority of cases) were accurate 98-99% of the time in both localized and remarkably even in metastatic samples. We also identified emergent properties of our lineage scores for tumor types on which the model was never trained (zero-shot learning). Adenocarcinoma/sarcoma lineage scores differentiated epithelioid from biphasic/sarcomatoid mesothelioma. Also, predicted lineage de-differentiation identified neuroendocrine/small cell tumors and was associated with poor outcomes across tumor types. Our platform-independent single-sample approach can be easily translated to existing RNA-seq platforms. ATLAS can complement and guide traditional histopathologic assessment in challenging situations and tumors of unknown primary.
PMID:38480799 | PMC:PMC10937974 | DOI:10.1038/s42003-024-05981-5
View details for PubMedID 38480799
-
More
-
Voxel-Level Dosimetry for Combined Iodine 131 Radiopharmaceutical Therapy and External Beam Radiation Therapy Treatment Paradigms for Head and Neck Cancer International journal of radiation oncology, biology, physics
Adam DP, Grudzinski JJ, Marsh IR, Hill PM, Cho SY, Bradshaw TJ, Longcor J, Burr A, Bruce JY, Harari PM, Bednarz BP
2024 Jul 15;119(4):1275-1284. doi: 10.1016/j.ijrobp.2024.02.005. Epub 2024 Feb 16.
-
More
PURPOSE: Targeted radiopharmaceutical therapy (RPT) in combination with external beam radiation therapy (EBRT) shows promise as a method to increase tumor control and mitigate potential high-grade toxicities associated with re-treatment for patients with recurrent head and neck cancer. This work establishes a patient-specific dosimetry framework that combines Monte Carlo-based dosimetry from the 2 radiation modalities at the voxel level using deformable image registration (DIR) and radiobiological constructs for patients enrolled in a phase 1 clinical trial combining EBRT and RPT.
METHODS AND MATERIALS: Serial single-photon emission computed tomography (SPECT)/computed tomography (CT) patient scans were performed at approximately 24, 48, 72, and 168 hours postinjection of 577.2 MBq/m2 (15.6 mCi/m2) CLR 131, an iodine 131-containing RPT agent. Using RayStation, clinical EBRT treatment plans were created with a treatment planning CT (TPCT). SPECT/CT images were deformably registered to the TPCT using the Elastix DIR module in 3D Slicer software and assessed by measuring mean activity concentrations and absorbed doses. Monte Carlo EBRT dosimetry was computed using EGSnrc. RPT dosimetry was conducted using RAPID, a GEANT4-based RPT dosimetry platform. Radiobiological metrics (biologically effective dose and equivalent dose in 2-Gy fractions) were used to combine the 2 radiation modalities.
RESULTS: The DIR method provided good agreement for the activity concentrations and calculated absorbed dose in the tumor volumes for the SPECT/CT and TPCT images, with a maximum mean absorbed dose difference of -11.2%. Based on the RPT absorbed dose calculations, 2 to 4 EBRT fractions were removed from patient EBRT treatments. For the combined treatment, the absorbed dose to target volumes ranged from 57.14 to 75.02 Gy. When partial volume corrections were included, the mean equivalent dose in 2-Gy fractions to the planning target volume from EBRT + RPT differed -3.11% to 1.40% compared with EBRT alone.
CONCLUSIONS: This work demonstrates the clinical feasibility of performing combined EBRT + RPT dosimetry on TPCT scans. Dosimetry guides treatment decisions for EBRT, and this work provides a bridge for the same paradigm to be implemented within the rapidly emerging clinical RPT space.
PMID:38367914 | DOI:10.1016/j.ijrobp.2024.02.005
View details for PubMedID 38367914
-
More
-
Immune Escape Strategies in Head and Neck Cancer: Evade, Resist, Inhibit, Recruit Cancers
Kostecki KL, Iida M, Crossman BE, Salgia R, Harari PM, Bruce JY, Wheeler DL
2024 Jan 11;16(2):312. doi: 10.3390/cancers16020312.
-
More
Head and neck cancers (HNCs) arise from the mucosal lining of the aerodigestive tract and are often associated with alcohol use, tobacco use, and/or human papillomavirus (HPV) infection. Over 600,000 new cases of HNC are diagnosed each year, making it the sixth most common cancer worldwide. Historically, treatments have included surgery, radiation, and chemotherapy, and while these treatments are still the backbone of current therapy, several immunotherapies have recently been approved by the Food and Drug Administration (FDA) for use in HNC. The role of the immune system in tumorigenesis and cancer progression has been explored since the early 20th century, eventually coalescing into the current three-phase model of cancer immunoediting. During each of the three phases-elimination, equilibrium, and escape-cancer cells develop and utilize multiple strategies to either reach or remain in the final phase, escape, at which point the tumor is able to grow and metastasize with little to no detrimental interference from the immune system. In this review, we summarize the many strategies used by HNC to escape the immune system, which include ways to evade immune detection, resist immune cell attacks, inhibit immune cell functions, and recruit pro-tumor immune cells.
PMID:38254801 | PMC:PMC10814769 | DOI:10.3390/cancers16020312
View details for PubMedID 38254801
-
More
-
Emerging Prognostic and Predictive Significance of Stress Keratin 17 in HPV-Associated and Non HPV-Associated Human Cancers: A Scoping Review Viruses
Lozar T, Wang W, Gavrielatou N, Christensen L, Lambert PF, Harari PM, Rimm DL, Burtness B, Kuhar CG, Carchman EH
2023 Nov 25;15(12):2320. doi: 10.3390/v15122320.
-
More
A growing body of literature suggests that the expression of cytokeratin 17 (K17) correlates with inferior clinical outcomes across various cancer types. In this scoping review, we aimed to review and map the available clinical evidence of the prognostic and predictive value of K17 in human cancers. PubMed, Web of Science, Embase (via Scopus), Cochrane Central Register of Controlled Trials, and Google Scholar were searched for studies of K17 expression in human cancers. Eligible studies were peer-reviewed, published in English, presented original data, and directly evaluated the association between K17 and clinical outcomes in human cancers. Of the 1705 studies identified in our search, 58 studies met criteria for inclusion. Studies assessed the prognostic significance (n = 54), predictive significance (n = 2), or both the prognostic and predictive significance (n = 2). Altogether, 11 studies (19.0%) investigated the clinical relevance of K17 in cancers with a known etiologic association to HPV; of those, 8 (13.8%) were focused on head and neck squamous cell carcinoma (HNSCC), and 3 (5.1%) were focused on cervical squamous cell carcinoma (SCC). To date, HNSCC, as well as triple-negative breast cancer (TNBC) and pancreatic cancer, were the most frequently studied cancer types. K17 had prognostic significance in 16/17 investigated cancer types and 43/56 studies. Our analysis suggests that K17 is a negative prognostic factor in the majority of studied cancer types, including HPV-associated types such as HNSCC and cervical cancer (13/17), and a positive prognostic factor in 2/17 studied cancer types (urothelial carcinoma of the upper urinary tract and breast cancer). In three out of four predictive studies, K17 was a negative predictive factor for chemotherapy and immune checkpoint blockade therapy response.
PMID:38140561 | PMC:PMC10748233 | DOI:10.3390/v15122320
View details for PubMedID 38140561
-
More
-
Combining Dual Checkpoint Immunotherapy with Ablative Radiation to All Sites of Oligometastatic Non-Small Cell Lung Cancer: Toxicity and Efficacy Results of a Phase 1b Trial International journal of radiation oncology, biology, physics
Bassetti MF, Morris BA, Sethakorn N, Lang JM, Schehr JL, Zhao SG, Morris ZS, Buehler D, Eickhoff JC, Harari PM, Traynor AM, Campbell TC, Baschnagel AM, Leal TA
2024 Apr 1;118(5):1481-1489. doi: 10.1016/j.ijrobp.2023.11.040. Epub 2023 Dec 8.
-
More
PURPOSE: Ablative local treatment of all radiographically detected metastatic sites in patients with oligometastatic non-small cell lung cancer (NSCLC) increases progression-free survival (PFS) and overall survival (OS). Prior studies demonstrated the safety of combining stereotactic body radiation therapy (SBRT) with single-agent immunotherapy. We investigated the safety of combining SBRT to all metastatic tumor sites with dual checkpoint, anticytotoxic T-lymphocyte-associated protein 4 (anti-CTLA-4), and anti-programmed cell death ligand 1 (anti-PD-L1) immunotherapy for patients with oligometastatic NSCLC.
METHODS AND MATERIALS: We conducted a phase 1b clinical trial in patients with oligometastatic NSCLC with up to 6 sites of extracranial metastatic disease. All sites of disease were treated with SBRT to a dose of 30 to 50 Gy in 5 fractions. Dual checkpoint immunotherapy was started 7 days after completion of radiation using anti-CTLA-4 (tremelimumab) and anti-PD-L1 (durvalumab) immunotherapy for a total of 4 cycles followed by durvalumab alone until progression or toxicity.
RESULTS: Of the 17 patients enrolled in this study, 15 patients received at least 1 dose of combination immunotherapy per protocol. The study was closed early (17 of planned 21 patients) due to slow accrual during the COVID-19 pandemic. Grade 3+ treatment-related adverse events were observed in 6 patients (40%), of which only one was possibly related to the addition of SBRT to immunotherapy. Median PFS was 42 months and median OS has not yet been reached.
CONCLUSIONS: Delivering ablative SBRT to all sites of metastatic disease in combination with dual checkpoint immunotherapy did not result in excessive rates of toxicity compared with historical studies of dual checkpoint immunotherapy alone. Although the study was not powered for treatment efficacy results, durable PFS and OS results suggest potential therapeutic benefit compared with immunotherapy or radiation alone in this patient population.
PMID:38072321 | PMC:PMC10947887 | DOI:10.1016/j.ijrobp.2023.11.040
View details for PubMedID 38072321
-
More
-
Author Correction: Microphysiological model reveals the promise of memory-like natural killer cell immunotherapy for HIV<sup>±</sup> cancer Nature communications
Ayuso JM, Farooqui M, Virumbrales-Muñoz M, Denecke K, Rehman S, Schmitz R, Guerrero JF, Sanchez-de-Diego C, Campo SA, Maly EM, Forsberg MH, Kerr SC, Striker R, Sherer NM, Harari PM, Capitini CM, Skala MC, Beebe DJ
2023 Nov 10;14(1):7292. doi: 10.1038/s41467-023-43057-w.
-
Microphysiological model reveals the promise of memory-like natural killer cell immunotherapy for HIV<sup>±</sup> cancer Nature communications
Ayuso JM, Farooqui M, Virumbrales-Muñoz M, Denecke K, Rehman S, Schmitz R, Guerrero JF, Sanchez-de-Diego C, Campo SA, Maly EM, Forsberg MH, Kerr SC, Striker R, Sherer NM, Harari PM, Capitini CM, Skala MC, Beebe DJ
2023 Oct 21;14(1):6681. doi: 10.1038/s41467-023-41625-8.
-
More
Numerous studies are exploring the use of cell adoptive therapies to treat hematological malignancies as well as solid tumors. However, there are numerous factors that dampen the immune response, including viruses like human immunodeficiency virus. In this study, we leverage human-derived microphysiological models to reverse-engineer the HIV-immune system interaction and evaluate the potential of memory-like natural killer cells for HIV+ head and neck cancer, one of the most common tumors in patients living with human immunodeficiency virus. Here, we evaluate multiple aspects of the memory-like natural killer cell response in human-derived bioengineered environments, including immune cell extravasation, tumor penetration, tumor killing, T cell dependence, virus suppression, and compatibility with retroviral medication. Overall, these results suggest that memory-like natural killer cells are capable of operating without T cell assistance and could simultaneously destroy head and neck cancer cells as well as reduce viral latency.
PMID:37865647 | PMC:PMC10590421 | DOI:10.1038/s41467-023-41625-8
View details for PubMedID 37865647
-
More
-
Stress Keratin 17 Is a Predictive Biomarker Inversely Associated with Response to Immune Check-Point Blockade in Head and Neck Squamous Cell Carcinomas and Beyond Cancers
Lozar T, Laklouk I, Golfinos AE, Gavrielatou N, Xu J, Flynn C, Keske A, Yu M, Bruce JY, Wang W, Kuhar CG, Bailey HH, Harari PM, Dinh HQ, Rimm DL, Hu R, Lambert PF, Fitzpatrick MB
2023 Oct 9;15(19):4905. doi: 10.3390/cancers15194905.
-
More
Low response rates in immune check-point blockade (ICB)-treated head and neck squamous cell carcinoma (HNSCC) drive a critical need for robust, clinically validated predictive biomarkers. Our group previously showed that stress keratin 17 (CK17) suppresses macrophage-mediated CXCL9/CXCL10 chemokine signaling involved in attracting activated CD8+ T cells into tumors, correlating with decreased response rate to pembrolizumab-based therapy in a pilot cohort of ICB-treated HNSCC (n = 26). Here, we performed an expanded analysis of the predictive value of CK17 in ICB-treated HNSCC according to the REMARK criteria and investigated the gene expression profiles associated with high CK17 expression. Pretreatment samples from pembrolizumab-treated HNSCC patients were stained via immunohistochemistry using a CK17 monoclonal antibody (n = 48) and subjected to spatial transcriptomic profiling (n = 8). Our findings were validated in an independent retrospective cohort (n = 22). CK17 RNA expression in pembrolizumab-treated patients with various cancer types was investigated for predictive significance. Of the 48 patients (60% male, median age of 61.5 years), 21 (44%) were CK17 high, and 27 (56%) were CK17 low. A total of 17 patients (35%, 77% CK17 low) had disease control, while 31 patients (65%, 45% CK17 low) had progressive disease. High CK17 expression was associated with a lack of disease control (p = 0.037), shorter time to treatment failure (p = 0.025), and progression-free survival (PFS, p = 0.004), but not overall survival (OS, p = 0.06). A high CK17 expression was associated with lack of disease control in an independent validation cohort (p = 0.011). PD-L1 expression did not correlate with CK17 expression or clinical outcome. CK17 RNA expression was predictive of PFS and OS in 552 pembrolizumab-treated cancer patients. Our findings indicate that high CK17 expression may predict resistance to ICB in HNSCC patients and beyond.
PMID:37835599 | PMC:PMC10571921 | DOI:10.3390/cancers15194905
View details for PubMedID 37835599
-
More
-
Long-term Outcomes of Bevacizumab and Chemoradiation for Locoregionally Advanced Nasopharyngeal Carcinoma: A Nonrandomized Controlled Trial JAMA network open
Lee NY, Harris J, Kim J, Garden A, Mechalakos J, Pfister DG, Chan TC, Hu K, Colevas AD, Frank S, Shenouda G, Bar-Ad V, Waldron JN, Harari PM, Raben A, Torres-Saavedra P, Le Q
2023 Jun 1;6(6):e2316094. doi: 10.1001/jamanetworkopen.2023.16094.
-
More
IMPORTANCE: The long-term outcomes associated with adding bevacizumab, a vascular endothelial growth factor inhibitor, to standard chemoradiation have continued to be favorable for a group of patients with locoregionally advanced nasopharyngeal carcinoma (NPC).
OBJECTIVE: To assess long-term toxic effects and clinical outcomes associated with chemotherapy, radiation therapy (RT), and bevacizumab for NPC.
DESIGN, SETTING, AND PARTICIPANTS: This single-arm phase II nonrandomized controlled trial was conducted by the National Cancer Trials Network group and NRG Oncology (formerly Radiation Therapy Oncology Group), with accrual from December 13, 2006, to February 5, 2009, and data analysis from June 26 to July 1, 2019. The study was conducted at 19 cancer centers with a median (IQR) follow-up of 9.0 (7.7-9.3) years. Included patients were adults (aged ≥18 years) with NPC that was World Health Organization (WHO) histologic grade I to IIb or III, American Joint Committee on Cancer stage IIB or greater, and with or without lymph node involvement.
INTERVENTIONS: Patients received 3 cycles of bevacizumab (15 mg/kg) concurrently with standard cisplatin (100 mg/m2) and RT (69.96 Gy) followed by 3 cycles of adjuvant bevacizumab (15 mg/kg) given concurrently with cisplatin (80 mg/m2) and fluorouracil (1000 mg/m2/d).
MAIN OUTCOMES AND MEASURES: The primary end point was grade 4 hemorrhage or grade 5 adverse events in the first year. Secondary end points were locoregional progression-free (LRPF) interval, distant metastasis-free (DMF) interval, progression-free survival (PFS), overall survival (OS), and other adverse events. Long-term toxic effects and clinical outcomes were reported due to the limited follow-up in the initial report for this trial and the importance of long-term outcomes when combining bevacizumab with chemoradiation.
RESULTS: Among 46 patients with NPC who were enrolled, 44 patients were analyzed (29 males [65.9%]; 23 Asian [52.3%], 2 Black [4.5%], and 16 White [36.4%]; 38 not Hispanic [86.4%]; median [IQR] age, 48.5 [39.0-56.0] years). There were 33 patients with a Zubrod performance status of 0, indicating that they were fully functional and asymptomatic (75.0%); 32 patients with a WHO histologic grade of IIb or III (72.7%); and 39 patients with stage III or IVB disease (88.6%). Among analyzed patients, 42 individuals received radiation therapy of 69.96 Gy or greater (95.5%; dose range, 65.72-70.00 Gy); 30 patients received 3 cycles of cisplatin (68.2%) with RT, and 31 patients received 3 cycles of bevacizumab with RT (70.5%); this was followed by 3 cycles of adjuvant cisplatin in 21 patients (47.7%), fluorouracil in 24 patients (54.5%), and bevacizumab in 23 patients (52.3%). No grade 4 hemorrhage or grade 5 AEs were reported in the first year or thereafter. Late grade 3 AEs occurred in 16 patients (36.4%), including 7 patients with dysphagia (15.9%), 6 patients with hearing impairment (13.6%), and 2 patients with dry mouth (4.5%). The 1- and 5-year rates of feeding tube use were 5 of 41 patients (12.2%) and 0 of 27 patients, respectively. There were 19 patients (43.2%) who progressed or died without disease progression (6 patients with locoregional progression [13.6%], 8 patients with distant progression [18.2%], and 5 patients who died without progression [11.4%]). The 5- and 7-year rates were 79.5% (95% CI, 67.6%-91.5%) and 69.7% (95% CI, 55.9%-83.5%) for OS, 61.2% (95% CI, 46.8%-75.6%) and 56.3% (95% CI, 41.5%-71.1%) for PFS, 74.9% (95% CI, 61.4%-86.6%) and 72.3% (95% CI, 58.4%-84.7%) for LRPF interval, and 79.5% (95% CI,66.4%-90.0%) for both times for DMF interval. Among 13 patients who died, death was due to disease in 8 patients (61.5%).
CONCLUSIONS AND RELEVANCE: In this nonrandomized controlled trial, no grade 4 hemorrhage or grade 5 AEs were reported in the first year or thereafter among patients with NPC receiving bevacizumab combined with chemoradiation. The rate of distant metastasis was low although 89% of patients had stage III to IVB disease, suggesting that further investigation may be warranted.
TRIAL REGISTRATION: ClinicalTrials.gov Identifier: NCT00408694.
PMID:37266942 | PMC:PMC10238946 | DOI:10.1001/jamanetworkopen.2023.16094
View details for PubMedID 37266942
-
More
-
Microphysiological head and neck cancer model identifies novel role of lymphatically secreted monocyte migration inhibitory factor in cancer cell migration and metabolism Biomaterials
Yada RC, Desa DE, Gillette AA, Bartels E, Harari PM, Skala MC, Beebe DJ, Kerr SC
2023 Jul;298:122136. doi: 10.1016/j.biomaterials.2023.122136. Epub 2023 Apr 27.
-
More
Regional metastasis of head and neck cancer (HNC) is prevalent (approximately 50% of patients at diagnosis), yet the underlying drivers and mechanisms of lymphatic spread remain unclear. The complex tumor microenvironment (TME) of HNC plays a crucial role in disease maintenance and progression; however, the contribution of the lymphatics remains underexplored. We created a primary patient cell derived microphysiological system that incorporates cancer-associated-fibroblasts from patients with HNC alongside a HNC tumor spheroid and a lymphatic microvessel to create an in vitro TME platform to investigate metastasis. Screening of soluble factor signaling identified novel secretion of macrophage migration inhibitory factor (MIF) by lymphatic endothelial cells conditioned in the TME. Importantly, we also observed patient-to-patient heterogeneity in cancer cell migration similar to the heterogeneity observed in clinical disease. Optical metabolic imaging at the single cell level identified a distinct metabolic profile of migratory versus non-migratory HNC cells in a microenvironment dependent manner. Additionally, we report a unique role of MIF in increasing HNC reliance on glycolysis over oxidative phosphorylation. This multicellular, microfluidic platform expands the tools available to explore HNC biology in vitro through multiple orthogonal outputs and establishes a system with enough resolution to visualize and quantify patient-to-patient heterogeneity.
PMID:37178589 | PMC:PMC10205684 | DOI:10.1016/j.biomaterials.2023.122136
View details for PubMedID 37178589
-
More
-
Creation of waterproof, TLD probes for dose measurements to validate image-based radiopharmaceutical therapy dosimetry workflow Biomedical physics & engineering express
Adam DP, Hammer C, Malyshev JZ, Culberson WS, Bradshaw TJ, Grudzinski JJ, Harari PM, Bednarz BP
2023 May 12;9(4):10.1088/2057-1976/accf22. doi: 10.1088/2057-1976/accf22.
-
More
Voxel-level dosimetry based on nuclear medicine images offers patient-specific personalization of radiopharmaceutical therapy (RPT) treatments. Clinical evidence is emerging demonstrating improvements in treatment precision in patients when voxel-level dosimetry is used compared to MIRD. Voxel-level dosimetry requires absolute quantification of activity concentrations in the patient, but images from SPECT/CT scanners are not quantitative and require calibration using nuclear medicine phantoms. While phantom studies can validate a scanner's ability to recover activity concentrations, these studies provide only a surrogate for the true metric of interest: absorbed doses. Measurements using thermoluminescent dosimeters (TLDs) are a versatile and accurate method of measuring absorbed dose. In this work, a TLD probe was manufactured that can fit into currently available nuclear medicine phantoms for the measurement of absorbed dose of RPT agents. Next, 748 MBq of I-131 was administered to a 16 ml hollow source sphere placed in a 6.4 L Jaszczak phantom in addition to six TLD probes, each holding 4 TLD-100 1 × 1 × 1 mm TLD-100 (LiF:Mg,Ti) microcubes. The phantom then underwent a SPECT/CT scan in accordance with a standard SPECT/CT imaging protocol for I-131. The SPECT/CT images were then input into a Monte Carlo based RPT dosimetry platform named RAPID and a three dimensional dose distribution in the phantom was estimated. Additionally, a GEANT4 benchmarking scenario (denoted 'idealized') was created using a stylized representation of the phantom. There was good agreement for all six probes, the differences between measurement and RAPID ranged between -5.5% and 0.9%. The difference between the measured and the idealized GEANT4 scenario was calculated and ranged from -4.3% and -20.5%. This work demonstrates good agreement between TLD measurements and RAPID. In addition, it introduces a novel TLD probe that can be easily introduced into clinical nuclear medicine workflows to provide QA of image-based dosimetry for RPT treatments.
PMID:37084718 | PMC:PMC11186108 | DOI:10.1088/2057-1976/accf22
View details for PubMedID 37084718
-
More
-
Targeting of Head and Neck Cancer by Radioiodinated CLR1404 in Murine Xenograft Tumor Models with Partial Volume Corrected Theranostic Dosimetry Cancer biotherapy & radiopharmaceuticals
Marsh IR, Li C, Grudzinski J, Jeffery J, Longhurst C, Adam DP, Hernandez R, Weichert JP, Harari PM, Bednarz BP
2023 Sep;38(7):458-467. doi: 10.1089/cbr.2022.0084. Epub 2023 Apr 5.
-
More
Background: Delivery of radiotherapeutic dose to recurrent head and neck cancer (HNC) is primarily limited by locoregional toxicity in conventional radiotherapy. As such, HNC patients stand to benefit from the conformal targeting of primary and remnant disease achievable with radiopharmaceutical therapies. In this study, the authors investigated the tumor targeting capacity of 131I-CLR1404 (iopofosine I-131) in various HNC xenograft mouse models and the impact of partial volume correction (PVC) on theranostic dosimetry based on 124I-CLR1404 (CLR 124) positron emission tomography (PET)/computed tomography (CT) imaging. Methods: Mice bearing flank tumor xenograft models of HNC (six murine cell line and six human patient derived) were intravenously administered 6.5-9.1 MBq of CLR 124 and imaged five times over the course of 6 d using microPET/CT. In vivo tumor uptake of CLR 124 was assessed and PVC for 124I was applied using a novel preclinical phantom. Using subject-specific theranostic dosimetry estimations for iopofosine I-131 based on CLR 124 imaging, a discrete radiation dose escalation study (2, 4, 6, and 8 Gy) was performed to evaluate tumor growth response to iopofosine I-131 relative to a single fraction of external beam radiation therapy (6 Gy). Results: PET imaging demonstrated consistent tumor selective uptake and retention of CLR 124 across all HNC xenograft models. Peak uptake of 4.4% ± 0.8% and 4.2% ± 0.4% was observed in squamous cell carcinoma-22B and UW-13, respectively. PVC application increased uptake measures by 47%-188% and reduced absolute differences between in vivo and ex vivo uptake measurements from 3.3% to 1.0 percent injected activity per gram. Tumor dosimetry averaged over all HNC models was 0.85 ± 0.27 Gy/MBq (1.58 ± 0.46 Gy/MBq with PVC). Therapeutic iopofosine I-131 studies demonstrated a variable, but linear relationship between iopofosine I-131 radiation dose and tumor growth delay (p < 0.05). Conclusions: Iopofosine I-131 demonstrated tumoricidal capacity in preclinical HNC tumor models and the theranostic pairing with CLR 124 presents a promising new treatment approach for personalizing administration of iopofosine I-131.
PMID:37022739 | PMC:PMC10516227 | DOI:10.1089/cbr.2022.0084
View details for PubMedID 37022739
-
More
-
Functional Outcomes After Transoral Plus Lateral Pharyngotomy Approach for Advanced Oral and Oropharyngeal Tumors OTO open
Colevas SM, Merfeld EC, Pflum ZE, Gessert TG, Wieland AM, Glazer TA, Burr AR, Harari PM, Hartig GK
2023 Feb 23;7(1):e35. doi: 10.1002/oto2.35. eCollection 2023 Jan-Mar.
-
More
OBJECTIVE: The aim of this study was to evaluate our institutional experience with the combined transoral plus lateral pharyngotomy (TO+LP) approach in a subset of patients with advanced or recurrent oral and oropharyngeal malignancy.
STUDY DESIGN: A retrospective study of procedures utilizing TO+LP for cancer resection between January 2007 and July 2019.
SETTING: Tertiary academic medical center.
METHODS: Thirty-one patients underwent a TO+LP approach for the resection of oral and oropharyngeal tumors. Functional and oncologic outcomes were analyzed.
RESULTS: Eighteen (58.1%) patients were treated with TO+LP for recurrent disease. Twenty-nine required free tissue transfer and 2 (6.5%) had positive margins. The median time to decannulation was 22 days (range 6-100 days). Thirteen (41.9%) patients still required enteral feeding at their most recent follow-up. Patients without a history of prior radiation were decannulated sooner (p = .009) and were less likely to require enteral feeding at the first postoperative follow-up (p = .034) than those who had prior head and neck radiotherapy.
CONCLUSION: A TO+LP approach can be used to achieve good functional and oncologic results for selected patients with advanced or recurrent oral and oropharyngeal cancer when minimally invasive options such as transoral robotic surgery, transoral laser microsurgery, or radiotherapy are not possible.
PMID:36998565 | PMC:PMC10046711 | DOI:10.1002/oto2.35
View details for PubMedID 36998565
-
More
-
Dual Axl/MerTK inhibitor INCB081776 creates a proinflammatory tumor immune microenvironment and enhances anti-PDL1 efficacy in head and neck cancer Head & neck
Kostecki KL, Iida M, Wiley AL, Kimani S, Mehall B, Tetreault K, Alexandridis R, Yu M, Hong S, Salgia R, Bruce JY, Birge RB, Harari PM, Wheeler DL
2023 May;45(5):1255-1271. doi: 10.1002/hed.27340. Epub 2023 Mar 20.
-
More
BACKGROUND: The tyrosine kinase receptors Axl and MerTK are highly overexpressed in head and neck cancer (HNC) cells, where they are critical drivers of survival, proliferation, metastasis, and therapeutic resistance.
METHODS: We investigated the role of Axl and MerTK in creating an immunologically "cold" tumor immune microenvironment (TIME) by targeting both receptors simultaneously with a small molecule inhibitor of Axl and MerTK (INCB081776). Effects of INCB081776 and/or anti-PDL1 on mouse oral cancer (MOC) cell growth and on the TIME were evaluated.
RESULTS: Targeting Axl and MerTK can reduce M2 and induce M1 macrophage polarization. In vivo, INCB081776 treatment alone or with anti-PDL1 appears to slow MOC tumor growth, increase proinflammatory immune infiltration, and decrease anti-inflammatory immune infiltration.
CONCLUSIONS: This data indicates that simultaneous targeting of Axl and MerTK with INCB081776, either alone or in combination with anti-PDL1, slows tumor growth and creates a proinflammatory TIME in mouse models of HNC.
PMID:36939040 | PMC:PMC10079616 | DOI:10.1002/hed.27340
View details for PubMedID 36939040
-
More
-
Interstitial Brachytherapy for Lip Cancer: Technical Aspects to Individualize Treatment Approach and Optimize Outcomes Practical radiation oncology
Merfeld EC, Witek ME, Francis DM, Burr AR, Wallace CR, Kuczmarska-Haas A, Lamichhane N, Kimple RJ, Glazer TA, Wieland AM, McCulloch TM, Hartig GK, Harari PM
2023 Jul-Aug;13(4):340-345. doi: 10.1016/j.prro.2023.01.004. Epub 2023 Jan 25.
-
More
Primary radiation therapy using interstitial brachytherapy (IBT) provides excellent local tumor control for early-stage squamous cell carcinoma of the lip. Technical aspects of treatment are important to optimize outcomes. In this report, we discuss patient selection criteria, procedural details, and dosimetric considerations for performing IBT for cancers of the lip. Catheters are inserted across the length of tumor entering and exiting approximately 5 mm beyond the palpable tumor extent. A custom mouthpiece is fabricated to facilitate normal tissue sparing. Patients undergo computed tomography imaging, the gross tumor volume is contoured based on physical examination and computed tomography findings, and an individualized brachytherapy plan is generated with the goals of achieving gross tumor volume D90% ≥ 90% and minimizing V150%. Ten patients with primary (n = 8) or recurrent (n = 2) cancers of the lip who received high-dose-rate lip IBT using 2.0- to 2.5-week treatment regimens are described (median prescription: 47.6 Gy in 14 fractions of 3.4 Gy). Local tumor control was 100%. There were no cases of acute grade ≥4 or late grade ≥2 toxicity, and cosmesis scores were graded as good to excellent in all patients. IBT represents an excellent treatment option for patients with lip squamous cell carcinoma. With careful attention to technical considerations furthered described in the present report, high rates of tumor control, low rates of toxicity, and favorable esthetic and functional outcomes can be achieved with IBT for lip cancer.
PMID:36709044 | PMC:PMC10330101 | DOI:10.1016/j.prro.2023.01.004
View details for PubMedID 36709044
-
More
-
Patterns of failure for hypopharynx cancer patients treated with limited high-dose radiotherapy treatment volumes Radiation oncology journal
Burr A, Harari P, Wieland A, Kimple R, Hartig G, Witek M
2022 Dec;40(4):225-231. doi: 10.3857/roj.2022.00311. Epub 2022 Dec 2.
-
More
PURPOSE: Optimal radiotherapy treatment volumes for patients with locally advanced hypopharynx squamous cell carcinoma should ensure maximal tumor coverage with minimal inclusion of normal surrounding structures. Here we evaluated the effectiveness of a direct 3-mm high-dose gross tumor volume to planning target volume expansion on clinical outcomes for hypopharynx cancers.
MATERIALS AND METHODS: We performed a retrospective analysis of patients with hypopharynx carcinoma treated between 2004 and 2018 with primary radiotherapy using a direct high-dose gross tumor volume to planning target volume expansion and with or without concurrent systemic therapy. Diagnostic imaging of recurrences was co-registered with the planning CT. Spatial and volumetric analyses of contoured recurrences were compared with planned isodose lines. Failures were initially defined as in field, marginal, elective nodal, and out of field. Each failure was further classified as central high-dose, peripheral high-dose, central intermediate/low-dose, peripheral intermediate/low-dose, and extraneous. Clinical outcomes were analyzed by Kaplan-Meier estimation.
RESULTS: Thirty-six patients were identified. At a median follow-up at 52.4 months, estimated 5-year overall survival was 59.3% (95% confidence interval [CI], 36.3%-74.1%), 5-year local and nodal control was 71.7% (95% CI, 47.1%-86.3%) and 69.9% (95% CI, 57.0%-82.6%), respectively. The most common failure was in the high-dose primary target volume. The gastrostomy tube retention rate at 1 year among patients without recurrence was 13.0% (95% CI, 3.2%-29.7%).
CONCLUSION: Minimal high-dose target volume expansions for hypopharynx cancers were associated with favorable locoregional control. This approach may enable therapy intensification to improve clinical outcomes.
PMID:36456541 | PMC:PMC9830040 | DOI:10.3857/roj.2022.00311
View details for PubMedID 36456541
-
More
-
Evolutionary Action Score of <em>TP53</em> Analysis in Pathologically High-Risk Human Papillomavirus-Negative Head and Neck Cancer From a Phase 2 Clinical Trial: NRG Oncology Radiation Therapy Oncology Group 0234 Advances in radiation oncology
Michikawa C, Torres-Saavedra PA, Silver NL, Harari PM, Kies MS, Rosenthal DI, Le Q, Jordan RC, Duose DY, Mallampati S, Trivedi S, Luthra R, Wistuba II, Osman AA, Lichtarge O, Foote RL, Parvathaneni U, Hayes DN, Pickering CR, Myers JN
2022 May 18;7(6):100989. doi: 10.1016/j.adro.2022.100989. eCollection 2022 Nov-Dec.
-
More
PURPOSE: An evolutionary action scoring algorithm (EAp53) based on phylogenetic sequence variations stratifies patients with head and neck squamous cell carcinoma (HNSCC) bearing TP53 missense mutations as high-risk, associated with poor outcomes, or low-risk, with similar outcomes as TP53 wild-type, and has been validated as a reliable prognostic marker. We performed this study to further validate prior findings demonstrating that EAp53 is a prognostic marker for patients with locally advanced HNSCC and explored its predictive value for treatment outcomes to adjuvant bio-chemoradiotherapy.
METHODS AND MATERIALS: Eighty-one resection samples from patients treated surgically for stage III or IV human papillomavirus-negative HNSCC with high-risk pathologic features, who received either radiation therapy + cetuximab + cisplatin (cisplatin) or radiation therapy + cetuximab + docetaxel (docetaxel) as adjuvant treatment in a phase 2 study were subjected to TP53 targeted sequencing and EAp53 scoring to correlate with clinical outcomes. Due to the limited sample size, patients were combined into 2 EAp53 groups: (1) wild-type or low-risk; and (2) high-risk or other.
RESULTS: At a median follow-up of 9.8 years, there was a significant interaction between EAp53 group and treatment for overall survival (P = .008), disease-free survival (P = .05), and distant metastasis (DM; P = .004). In wild-type or low-risk group, the docetaxel arm showed significantly better overall survival (hazard ratio [HR] 0.11, [0.03-0.36]), disease-free survival (HR 0.24, [0.09-0.61]), and less DM (HR 0.04, [0.01-0.31]) than the cisplatin arm. In high-risk or other group, differences between treatments were not statistically significant.
CONCLUSIONS: The docetaxel arm was associated with better survival than the cisplatin arm for patients with wild-type or low-risk EAp53. These benefits appear to be largely driven by a reduction in DM.
PMID:36420184 | PMC:PMC9677209 | DOI:10.1016/j.adro.2022.100989
View details for PubMedID 36420184
-
More
-
A clinical-grade liquid biomarker detects neuroendocrine differentiation in prostate cancer The Journal of clinical investigation
Zhao SG, Sperger JM, Schehr JL, McKay RR, Emamekhoo H, Singh A, Schultz ZD, Bade RM, Stahlfeld CN, Gilsdorf CS, Hernandez CI, Wolfe SK, Mayberry RD, Krause HM, Bootsma M, Helzer KT, Rydzewski N, Bakhtiar H, Shi Y, Blitzer G, Kyriakopoulos CE, Kosoff D, Wei XX, Floberg J, Sethakorn N, Sharifi M, Harari PM, Huang W, Beltran H, Choueiri TK, Scher HI, Rathkopf DE, Halabi S, Armstrong AJ, Beebe DJ, Yu M, Sundling KE, Taplin M, Lang JM
2022 Nov 1;132(21):e161858. doi: 10.1172/JCI161858.
-
More
BackgroundNeuroendocrine prostate cancer (NEPC) is an aggressive subtype, the presence of which changes the prognosis and management of metastatic prostate cancer.MethodsWe performed analytical validation of a Circulating Tumor Cell (CTC) multiplex RNA qPCR assay to identify the limit of quantification (LOQ) in cell lines, synthetic cDNA, and patient samples. We next profiled 116 longitudinal samples from a prospectively collected institutional cohort of 17 patients with metastatic prostate cancer (7 NEPC, 10 adenocarcinoma) as well as 265 samples from 139 patients enrolled in 3 adenocarcinoma phase II trials of androgen receptor signaling inhibitors (ARSIs). We assessed a NEPC liquid biomarker via the presence of neuroendocrine markers and the absence of androgen receptor (AR) target genes.ResultsUsing the analytical validation LOQ, liquid biomarker NEPC detection in the longitudinal cohort had a per-sample sensitivity of 51.35% and a specificity of 91.14%. However, when we incorporated the serial information from multiple liquid biopsies per patient, a unique aspect of this study, the per-patient predictions were 100% accurate, with a receiver-operating-curve (ROC) AUC of 1. In the adenocarcinoma ARSI trials, the presence of neuroendocrine markers, even while AR target gene expression was retained, was a strong negative prognostic factor.ConclusionOur analytically validated CTC biomarker can detect NEPC with high diagnostic accuracy when leveraging serial samples that are only feasible using liquid biopsies. Patients with expression of NE genes while retaining AR-target gene expression may indicate the transition to neuroendocrine differentiation, with clinical characteristics consistent with this phenotype.FundingNIH (DP2 OD030734, 1UH2CA260389, R01CA247479, and P30 CA014520), Department of Defense (PC190039 and PC200334), and Prostate Cancer Foundation (Movember Foundation - PCF Challenge Award).
PMID:36317634 | PMC:PMC9621140 | DOI:10.1172/JCI161858
View details for PubMedID 36317634
-
More
-
Identification of phenocopies improves prediction of targeted therapy response over DNA mutations alone NPJ genomic medicine
Bakhtiar H, Helzer KT, Park Y, Chen Y, Rydzewski NR, Bootsma ML, Shi Y, Harari PM, Sharifi M, Sjöström M, Lang JM, Yu M, Zhao SG
2022 Oct 17;7(1):58. doi: 10.1038/s41525-022-00328-7.
-
More
DNA mutations in specific genes can confer preferential benefit from drugs targeting those genes. However, other molecular perturbations can "phenocopy" pathogenic mutations, but would not be identified using standard clinical sequencing, leading to missed opportunities for other patients to benefit from targeted treatments. We hypothesized that RNA phenocopy signatures of key cancer driver gene mutations could improve our ability to predict response to targeted therapies, despite not being directly trained on drug response. To test this, we built gene expression signatures in tissue samples for specific mutations and found that phenocopy signatures broadly increased accuracy of drug response predictions in-vitro compared to DNA mutation alone, and identified additional cancer cell lines that respond well with a positive/negative predictive value on par or better than DNA mutations. We further validated our results across four clinical cohorts. Our results suggest that routine RNA sequencing of tumors to identify phenocopies in addition to standard targeted DNA sequencing would improve our ability to accurately select patients for targeted therapies in the clinic.
PMID:36253482 | PMC:PMC9576758 | DOI:10.1038/s41525-022-00328-7
View details for PubMedID 36253482
-
More
-
Targeting HER3-dependent activation of nuclear AKT improves radiotherapy of non-small cell lung cancer Radiotherapy and oncology : journal of the European Society for Therapeutic Radiology and Oncology
Toulany M, Iida M, Lettau K, Coan JP, Rebholz S, Khozooei S, Harari PM, Wheeler DL
2022 Sep;174:92-100. doi: 10.1016/j.radonc.2022.07.008. Epub 2022 Jul 15.
-
More
BACKGROUND: AKT1 must be present and activated in the nucleus immediately after irradiation to stimulate AKT1-dependent double-strand breaks (DSB) repair through the fast non-homologous end-joining (NHEJ) repair process. We investigated the subcellular distribution of AKT1 and the role of HER family receptor members on the phosphorylation of nuclear AKT and radiation response.
MATERIALS AND METHODS: Using genetic approaches and pharmacological inhibitors, we investigated the subcellular distribution of AKT1 and the role of HER family receptor members on the activation of nuclear AKT in non-small cell lung cancer (NSCLC) cells in vitro. ɤH2AX foci assay was applied to investigate the role of AKT activating signaling pathway on DSB repair. A mouse tumor xenograft model was used to study the impact of discovered signaling pathway activating nuclear AKT on the radiation response of tumors in vivo.
RESULTS: Our data suggests that neither ionizing radiation (IR) nor stimulation with HER family receptor ligands induced rapid nuclear translocation of endogenous AKT1. GFP-tagged exogenous AKT1 translocated to the nucleus under un-irradiated conditions and IR did not stimulate this translocation. Nuclear translocation of GFP-AKT1 was impaired by the AKT inhibitor MK2206 as shown by its accumulation in the cytoplasmic fraction. IR-induced phosphorylation of nuclear AKT was primarily dependent on HER3 expression and tyrosine kinase activation of epidermal growth factor receptor. In line with the role of AKT1 in DSB repair, the HER3 neutralizing antibody patritumab as well as HER3-siRNA diminished DSB repair in vitro. Combination of patritumab with radiotherapy improved the effect of radiotherapy on tumor growth delay in a xenograft model.
CONCLUSION: IR-induced activation of nuclear AKT occurs inside the nucleus that is mainly dependent on HER3 expression in NSCLC. These findings suggest that targeting HER3 in combination with radiotherapy may provide a logical treatment option for investigation in selected NSCLC patients.
PMID:35839938 | PMC:PMC10083767 | DOI:10.1016/j.radonc.2022.07.008
View details for PubMedID 35839938
-
More
-
Stress Keratin 17 Expression in Head and Neck Cancer Contributes to Immune Evasion and Resistance to Immune-Checkpoint Blockade Clinical cancer research : an official journal of the American Association for Cancer Research
Wang W, Lozar T, Golfinos AE, Lee D, Gronski E, Ward-Shaw E, Hayes M, Bruce JY, Kimple RJ, Hu R, Harari PM, Xu J, Keske A, Sondel PM, Fitzpatrick MB, Dinh HQ, Lambert PF
2022 Jul 1;28(13):2953-2968. doi: 10.1158/1078-0432.CCR-21-3039.
-
More
PURPOSE: We investigated whether in human head and neck squamous cell carcinoma (HNSCC) high levels of expression of stress keratin 17 (K17) are associated with poor survival and resistance to immunotherapy.
EXPERIMENTAL DESIGN: We investigated the role of K17 in regulating both the tumor microenvironment and immune responsiveness of HNSCC using a syngeneic mouse HNSCC model, MOC2. MOC2 gives rise to immunologically cold tumors that are resistant to immune-checkpoint blockade (ICB). We engineered multiple, independent K17 knockout (KO) MOC2 cell lines and monitored their growth and response to ICB. We also measured K17 expression in human HNSCC of patients undergoing ICB.
RESULTS: MOC2 tumors were found to express K17 at high levels. When knocked out for K17 (K17KO MOC2), these cells formed tumors that grew slowly or spontaneously regressed and had a high CD8+ T-cell infiltrate in immunocompetent syngeneic C57BL/6 mice compared with parental MOC2 tumors. This phenotype was reversed when we depleted mice for T cells. Whereas parental MOC2 tumors were resistant to ICB treatment, K17KO MOC2 tumors that did not spontaneously regress were eliminated upon ICB treatment. In a cohort of patients with HNSCC receiving pembrolizumab, high K17 expression correlated with poor response. Single-cell RNA-sequencing analysis revealed broad differences in the immune landscape of K17KO MOC2 tumors compared with parental MOC2 tumors, including differences in multiple lymphoid and myeloid cell types.
CONCLUSIONS: We demonstrate that K17 expression in HNSCC contributes to immune evasion and resistance to ICB treatment by broadly altering immune landscapes of tumors.
PMID:35621713 | PMC:PMC9250640 | DOI:10.1158/1078-0432.CCR-21-3039
View details for PubMedID 35621713
-
More
-
Longitudinal Molecular Profiling of Circulating Tumor Cells in Metastatic Renal Cell Carcinoma Journal of clinical oncology : official journal of the American Society of Clinical Oncology
Bootsma M, McKay RR, Emamekhoo H, Bade RM, Schehr JL, Mannino MC, Singh A, Wolfe SK, Schultz ZD, Sperger J, Xie W, Signoretti S, Kyriakopoulos CE, Kosoff D, Abel EJ, Helzer KT, Rydzewski N, Bakhtiar H, Shi Y, Blitzer G, Bassetti M, Floberg J, Yu M, Sethakorn N, Sharifi M, Harari PM, Choueiri TK, Lang JM, Zhao SG
2022 Nov 1;40(31):3633-3641. doi: 10.1200/JCO.22.00219. Epub 2022 May 26.
-
More
PURPOSE: Liquid biopsies in metastatic renal cell carcinoma (mRCC) provide a unique approach to understand the molecular basis of treatment response and resistance. This is particularly important in the context of immunotherapies, which target key immune-tumor interactions. Unlike metastatic tissue biopsies, serial real-time profiling of mRCC is feasible with our noninvasive circulating tumor cell (CTC) approach.
METHODS: We collected 457 longitudinal liquid biopsies from 104 patients with mRCC enrolled in one of two studies, either a prospective cohort or a phase II multicenter adaptive immunotherapy trial. Using a novel CTC capture and automated microscopy platform, we profiled CTC enumeration and expression of HLA I and programmed cell death-ligand 1 (PD-L1). Given their diametric immunological roles, we focused on the HLA I to PD-L1 ratio (HP ratio).
RESULTS: Patients with radiographic responses showed significantly lower CTC abundances throughout treatment. Furthermore, patients whose CTC enumeration trajectory was in the highest quartile (> 0.12 CTCs/mL annually) had shorter overall survival (median 17.0 months v 21.1 months, P < .001). Throughout treatment, the HP ratio decreased in patients receiving immunotherapy but not in patients receiving tyrosine kinase inhibitors. Patients with an HP ratio trajectory in the highest quartile (≥ 0.0012 annually) displayed significantly shorter overall survival (median 18.4 months v 21.2 months, P = .003).
CONCLUSION: In the first large longitudinal CTC study in mRCC to date to our knowledge, we identified the prognostic importance of CTC enumeration and the change over time of both CTC enumeration and the HP ratio. These insights into changes in both tumor burden and the molecular profile of tumor cells in response to different treatments provide potential biomarkers to predict and monitor response to immunotherapy in mRCC.
PMID:35617646 | PMC:PMC9622626 | DOI:10.1200/JCO.22.00219
View details for PubMedID 35617646
-
More
-
Validation of Monte Carlo <sup>131</sup> I radiopharmaceutical dosimetry workflow using a 3D-printed anthropomorphic head and neck phantom Medical physics
Adam DP, Grudzinski JJ, Bormett I, Cox BL, Marsh IR, Bradshaw TJ, Harari PM, Bednarz BP
2022 Aug;49(8):5491-5503. doi: 10.1002/mp.15699. Epub 2022 Jun 6.
-
More
PURPOSE: Approximately 50% of head and neck cancer (HNC) patients will experience loco-regional disease recurrence following initial courses of therapy. Retreatment with external beam radiotherapy (EBRT) is technically challenging and may be associated with a significant risk of irreversible damage to normal tissues. Radiopharmaceutical therapy (RPT) is a potential method to treat recurrent HNC in conjunction with EBRT. Phantoms are used to calibrate and add quantification to nuclear medicine images, and anthropomorphic phantoms can account for both the geometrical and material composition of the head and neck. In this study, we present the creation of an anthropomorphic, head and neck, nuclear medicine phantom, and its characterization for the validation of a Monte Carlo, SPECT image-based, 131 I RPT dosimetry workflow.
METHODS: 3D-printing techniques were used to create the anthropomorphic phantom from a patient CT dataset. Three 131 I SPECT/CT imaging studies were performed using a homogeneous, Jaszczak, and an anthropomorphic phantom to quantify the SPECT images using a GE Optima NM/CT 640 with a high energy general purpose collimator. The impact of collimator detector response (CDR) modeling and volume-based partial volume corrections (PVCs) upon the absorbed dose was calculated using an image-based, Geant4 Monte Carlo RPT dosimetry workflow and compared against a ground truth scenario. Finally, uncertainties were quantified in accordance with recent EANM guidelines.
RESULTS: The 3D-printed anthropomorphic phantom was an accurate re-creation of patient anatomy including bone. The extrapolated Jaszczak recovery coefficients were greater than that of the 3D-printed insert (∼22.8 ml) for both the CDR and non-CDR cases (with CDR: 0.536 vs. 0.493, non-CDR: 0.445 vs. 0.426, respectively). Utilizing Jaszczak phantom PVCs, the absorbed dose was underpredicted by 0.7% and 4.9% without and with CDR, respectively. Utilizing anthropomorphic phantom recovery coefficient overpredicted the absorbed dose by 3% both with and without CDR. All dosimetry scenarios that incorporated PVC were within the calculated uncertainty of the activity. The uncertainties in the cumulative activity ranged from 23.6% to 106.4% for Jaszczak spheres ranging in volume from 0.5 to 16 ml.
CONCLUSION: The accuracy of Monte Carlo-based dosimetry for 131 I RPT in HNC was validated with an anthropomorphic phantom. In this study, it was found that Jaszczak-based PVCs were sufficient. Future applications of the phantom could involve 3D printing and characterizing patient-specific volumes for more personalized RPT dosimetry estimates.
PMID:35607296 | PMC:PMC9388595 | DOI:10.1002/mp.15699
View details for PubMedID 35607296
-
More
-
Prospective Study of PET/MRI Tumor Response During Chemoradiotherapy for Patients With Low-risk and Intermediate-risk p16-positive Oropharynx Cancer American journal of clinical oncology
Witek ME, Kimple RJ, Avey GD, Burr AR, Chandereng T, Yu M, Hu R, Wieland AM, Labby ZE, Bruce JY, Brower JV, Hartig GK, Harari PM
2022 May 1;45(5):202-207. doi: 10.1097/COC.0000000000000910. Epub 2022 Apr 12.
-
More
OBJECTIVE: The objective of this study was to examine tumor response with positron emission tomography (PET)/magnetic resonance imaging (MRI) during chemoradiotherapy as a predictor of outcome in patients with p16-positive oropharynx cancer.
MATERIALS AND METHODS: Patients with p16-positive oropharynx cancer were treated with chemoradiotherapy. Low-risk (LR) disease was defined as T1-T3 and N0-2b and ≤10 pack-years and intermediate-risk (IR) disease as T4 or N2c-3 or >10 pack-years. Patients underwent a PET/MRI scan pretreatment and at fraction 10. Change in value of imaging means were analyzed by analysis of variance. K-means clustering with Euclidean distance functions were used for patient clustering. Silhouette width was used to determine the optimal number of clusters. Linear regression was performed on all radiographic metrics using patient and disease characteristics.
RESULTS: Twenty-four patients were enrolled with 7 LR and 11 IR patients available for analysis. Pretreatment imaging characteristics between LR and IR patients were similar. Patients with LR disease exhibited a larger reduction in maximum standardized uptake value (SUV) compared with IR patients (P<0.05). Cluster analysis defined 2 cohorts that exhibited a similar intratreatment response. Cluster 1 contained 7 of 7 LR patients and 8 of 11 IR patients. Cluster 2 contained 3 of 11 IR patients. Cluster 2 exhibited significant differences compared with cluster 1 in the change in primary tumor peak SUV and largest lymph node median SUV.
CONCLUSIONS: We identified that IR p16-positive oropharynx cancers exhibit heterogeneity in their PET/MRI response to chemoradiotherapy. These data support further study of intratreatment imaging response as a potential mechanism to identify patients with IR oropharynx cancer suitable for treatment deintensification.
PMID:35446279 | PMC:PMC9623610 | DOI:10.1097/COC.0000000000000910
View details for PubMedID 35446279
-
More
-
Nodal Metastasis Count and Oncologic Outcomes in Head and Neck Cancer: A Secondary Analysis of NRG/RTOG 9501, NRG/RTOG 0234, and EORTC 22931 International journal of radiation oncology, biology, physics
Lu DJ, Luu M, Gay C, Nguyen AT, Anderson EM, Bernier J, Cooper JS, Harari PM, Torres-Saavedra PA, Le Q, Chen MM, Clair JM, Ho AS, Zumsteg ZS
2022 Jul 15;113(4):787-795. doi: 10.1016/j.ijrobp.2022.03.033. Epub 2022 Apr 6.
-
More
PURPOSE: A better understanding of the relationship between the spread of head and neck squamous cell carcinoma (HNSCC) to regional lymph nodes (LNs) and the frequency and manner of treatment failure should help design better treatment intensification strategies. In this study, we evaluated the relationship between recurrence patterns, mortality, and number of pathologically positive (+) LNs in HNSCC in 3 prospective randomized controlled trials.
METHODS AND MATERIALS: We performed a secondary analysis of 947 patients with HNSCC enrolled in RTOG 9501 (n = 410), RTOG 0234 (n = 203), and EORTC 22931 (n = 334) undergoing surgery and postoperative radiation ± systemic therapy. Multivariable models were constructed for overall survival (OS), disease-free survival (DFS), locoregional relapse (LRR), and distant metastases (DM). Restricted cubic splines were used to model the nonlinear relationship between +LN number and outcomes.
RESULTS: In multivariable analysis, OS and DFS decreased with each +LN without plateau, most pronounced up to 5 +LNs (OS: hazard ratio [HR], 1.21 per +LN; 95% confidence interval [CI], 1.10-1.34; P < .001; DFS: HR per +LN, 1.19; 95% CI, 1.08-1.30; P < .001) and more gradually beyond this (OS: HR per +LN, 1.02; 95% CI, 1.01-1.06; P < .001; DFS: HR per +LN, 1.04; 95% CI, 1.02-1.06; P < .001). In contrast to LRR risk, which increased sharply up to 5 +LNs (HR per +LN, 1.28; 95% CI, 1.10-1.50; P < .001) but plateaued beyond this (HR per +LN, 1.00; 95% CI, 0.96-1.04; P = .98), DM risk increased continuously with increasing +LNs (≤5 +LNs: HR per +LN, 1.10; 95% CI, 1.01-1.20; P = .04; >5 +LNs: HR per +LN, 1.05; 95% CI, 1.02-1.08; P = .003).
CONCLUSIONS: In high-risk resected HNSCC, increased mortality was associated with increased +LN count. LRR and DM risk both increased in parallel up to 5 +LNs, but only DM continued to increase for further +LN increases. These differing recurrence patterns can help inform design of future treatments.
PMID:35395358 | PMC:PMC9583684 | DOI:10.1016/j.ijrobp.2022.03.033
View details for PubMedID 35395358
-
More
-
The Model of an ASTRO Servant Leader International journal of radiation oncology, biology, physics
DeWeese TL, Beyer D, Gunderson LL, Haffty BG, Harari PM, Lawton CA, Minsky BD, Steinberg ML
2021 Dec 1;111(5):1120-1121. doi: 10.1016/j.ijrobp.2021.08.014.
-
Defining high-risk elective contralateral neck radiation volumes for oropharynx cancer Head & neck
Witek ME, Woody NM, Musunuru HB, Hill PM, Yadav P, Burr AR, Ko HC, Ross RB, Kimple RJ, Harari PM
2022 Feb;44(2):317-324. doi: 10.1002/hed.26924. Epub 2021 Nov 11.
-
More
BACKGROUND: To define the location of the initial contralateral lymph node (LN) metastasis in patients with oropharynx cancer.
METHODS: The location of the LN centroids from patients with oropharynx cancer and a single radiographically positive contralateral LN was defined. A clinical target volume (CTV) inclusive of all LN centroids was created, and its impact on dose to organs at risk was assessed.
RESULTS: We identified 55 patients of which 49/55 had a single contralateral LN in level IIA, 4/55 in level III, 1/55 in level IIB, and 1/55 in the retropharynx. Mean radiation dose to the contralateral parotid gland was 15.1 and 21.0 Gy, (p <0.001) using the modeled high-risk elective CTV and a consensus CTV, respectively.
CONCLUSIONS: We present a systematic approach for identifying the contralateral nodal regions at highest risk of harboring subclinical disease in patients with oropharynx cancer that warrants prospective clinical study.
PMID:34761832 | PMC:PMC9723806 | DOI:10.1002/hed.26924
View details for PubMedID 34761832
-
More
-
Primary head and neck tumour-derived fibroblasts promote lymphangiogenesis in a lymphatic organotypic co-culture model EBioMedicine
Lugo-Cintrón KM, Ayuso JM, Humayun M, Gong MM, Kerr SC, Ponik SM, Harari PM, Virumbrales-Muñoz M, Beebe DJ
2021 Nov;73:103634. doi: 10.1016/j.ebiom.2021.103634. Epub 2021 Oct 18.
-
More
BACKGROUND: In head and neck cancer, intratumour lymphatic density and tumour lymphangiogenesis have been correlated with lymphatic metastasis, making lymphangiogenesis a promising therapeutic target. However, inter-patient tumour heterogeneity makes it challenging to predict tumour progression and lymph node metastasis. Understanding the lymphangiogenic-promoting factors leading to metastasis (e.g., tumour-derived fibroblasts or TDF), would help develop strategies to improve patient outcomes.
METHODS: A microfluidic in vitro model of a tubular lymphatic vessel was co-cultured with primary TDF from head and neck cancer patients to evaluate the effect of TDF on lymphangiogenesis. We assessed the length and number of lymphangiogenic sprouts and vessel permeability via microscopy and image analysis. Finally, we characterised lymphatic vessel conditioning by TDF via RT-qPCR.
FINDINGS: Lymphatic vessels were conditioned by the TDF in a patient-specific manner. Specifically, the presence of TDF induced sprouting, altered vessel permeability, and increased the expression of pro-lymphangiogenic genes. Gene expression and functional responses in the fibroblast-conditioned lymphatic vessels were consistent with the patient tumour stage and lymph node status. IGF-1, upregulated among patients, was targeted to validate our personalised medicine approach. Interestingly, IGF-1 blockade was not effective across different patients.
INTERPRETATION: The use of lymphatic organotypic models incorporating head and neck TDF provides insight into the pathways leading to lymphangiogenesis in each patient. This model provided a platform to test anti-angiogenic therapeutics and inform of their effectiveness for individual patients.
FUNDING: NIH R33CA225281. Wisconsin Head and Neck SPORE NIH P50DE026787. NIH R01AI34749.
PMID:34673450 | PMC:PMC8528684 | DOI:10.1016/j.ebiom.2021.103634
View details for PubMedID 34673450
-
More
-
Why an Increasing Number of Unmatched Residency Positions in Radiation Oncology? A Survey of Fourth-Year Medical Students Advances in radiation oncology
Blitzer GC, Parekh AD, Chen S, Taparra K, Kahn JM, Fields EC, Stahl JM, Rosenberg SA, Buatti JM, Laucis AM, Wang Y, Mayhew DL, McDonald AM, Harari PM, Brower JV
2021 Jun 24;6(5):100743. doi: 10.1016/j.adro.2021.100743. eCollection 2021 Sep-Oct.
-
More
PURPOSE: The number of US fourth-year medical students applying to radiation oncology has decreased during the past few years. We conducted a survey of fourth-year medical students to examine factors that may be influencing the decision to pursue radiation oncology.
METHODS AND MATERIALS: An anonymous online survey was sent to medical students at 9 participating US medical schools.
RESULTS: A total of 232 medical students completed the survey. Of the 153 students who stated they were never interested in radiation oncology, 77 (50%) reported never having been exposed to the specialty as their reason for not pursuing radiation oncology. The job market was the most commonly cited factor among students who said they were once interested in but ultimately chose not to pursue radiation oncology. Conversely, the recent low pass rates for board examinations and a perception of a lack of diversity within radiation oncology had the least influence.
CONCLUSIONS: Despite discussion of potential measures to address this disquieting trend, there have been minimal formal attempts to characterize and address potential causes of a decreasing interest in radiation oncology. This study's data are consistent with previous research regarding the trend of decreased medical student interest in radiation oncology and may be used as part of ongoing introspective assessment to inform future change within radiation oncology.
PMID:34466713 | PMC:PMC8385400 | DOI:10.1016/j.adro.2021.100743
View details for PubMedID 34466713
-
More
-
Opioid use in patients undergoing treatment for oral cavity cancer Journal of pain management
Ko HC, Mehra MN, Burr AR, Wieland AM, Kimple RJ, Hartig GK, Harari PM, Witek ME
2020;13(2):167-173.
-
More
OBJECTIVE: In the context of the opioid epidemic, there is value in examining the use of opioids in specific cancer patient cohorts. We analyzed opioid use in patients undergoing adjuvant therapy for oral cavity cancer to define the incidence of new persistent use beyond 3 months.
STUDY DESIGN: Retrospective.
SETTING: Comprehensive academic cancer center.
SUBJECTS AND METHODS: We performed a retrospective IRB-approved analysis of opioid use in patients who received adjuvant radiotherapy with or with concurrent systemic therapy for surgically resected oral cavity cancer between 2003 and 2016. Factors associated with opioid use were evaluated by Chi-square test and one-way ANOVA. The Kaplan-Meier method was used to estimate overall survival.
RESULTS: Of 77 identified patients, 10 (13%) patients received opioid prescriptions at 3 months or greater following completion of radiotherapy. Patients who were opioid naive prior to surgery required significantly fewer opioid prescriptions than intermittent or chronic opioid users. No specific factors were associated with new persistent opioid use.
CONCLUSIONS: Patients undergoing surgery and adjuvant radiotherapy for oral cavity cancer who required opioids for cancer treatment related pain are at minimal risk for new dependency. Judicious pain management should be applied for patients with a history of prior opioid use. Larger patient cohorts will be needed to identify patient, disease, and treatment characteristics associated with new persistent use given its limited incidence.
PMID:34457108 | PMC:PMC8388255
View details for PubMedID 34457108
-
More
-
Combining Stereotactic Body Radiotherapy and Microwave Ablation Appears Safe and Feasible for Renal Cell Carcinoma in an Early Series Clinical genitourinary cancer
Blitzer GC, Wojcieszynski A, Abel EJ, Best S, Lee FT, Hinshaw JL, Wells S, Ziemlewicz TJ, Lubner MG, Alexander M, Yadav P, Bayouth JE, Floberg J, Cooley G, Harari PM, Bassetti MF
2021 Oct;19(5):e313-e318. doi: 10.1016/j.clgc.2021.04.010. Epub 2021 Apr 20.
-
More
Microwave (MW) ablation and stereotactic body radiation therapy (SBRT) are both used in treating inoperable renal cell carcinoma (RCC). MW ablation and SBRT have potentially complementary advantages and limitations. Combining SBRT and MW ablation may optimize tumor control and toxicity for patients with larger (> 5 cm) RCCs or those with vascular involvement. Seven patients with RCC were treated at our institution with combination of SBRT and MW ablation, median tumor size of 6.4 cm. Local control was 100% with a median follow-up of 15 months. Four patients experienced grade 2 nausea during SBRT. Three patients experienced toxicities after MW ablation, 2 with grade 1 hematuria and 1 with grade 3 retroperitoneal bleed/collecting system injury. Median eGFR (estimated glomerular filtration rate) preceding and following SBRT and MW ablation was 69 mL/min/1.73 m2 and 68 mL/min/1.73 m2 (P = .19), respectively. In patients who are not surgical candidates, larger RCCs or those with vascular invasion are challenging to treat. Combination treatment with SBRT and MW ablation may balance the risks and benefits of both therapies and demonstrates high local control in our series. MW ablation and SBRT have potentially complementary advantages and limitations.
PMID:34024743 | DOI:10.1016/j.clgc.2021.04.010
View details for PubMedID 34024743
-
More
-
Declining Medical Student Interest in Radiation Oncology: Wake-Up Call With a Silver Lining? International journal of radiation oncology, biology, physics
Brower JV, Blitzer GC, Vapiwala N, Harari PM
2021 Jun 1;110(2):274-277. doi: 10.1016/j.ijrobp.2021.02.035. Epub 2021 Mar 12.
-
Refining Guidelines Regarding Unilateral Treatment in Patients With Well-lateralized Squamous Cell Carcinoma of the Palatine Tonsil and Multiple Positive Nodes or Extranodal Extension Practical radiation oncology
Amdur RJ, Harari PM, Dziegielewski PT, Mendenhall WM
2021 May-Jun;11(3):e247-e251. doi: 10.1016/j.prro.2020.11.011. Epub 2021 Jan 9.
-
Treating Advanced Head and Neck Cancer When Cisplatin Is Not an Option Journal of clinical oncology : official journal of the American Society of Clinical Oncology
Jhawar SR, Bonomi M, Harari PM
2021 Jan 1;39(1):7-12. doi: 10.1200/JCO.20.02720. Epub 2020 Dec 4.
-
More
The Oncology Grand Rounds series is designed to place original reports published in the Journal into clinical context. A case presentation is followed by a description of diagnostic and management challenges, a review of the relevant literature, and a summary of the authors' suggested management approaches. The goal of this series is to help readers better understand how to apply the results of key studies, including those published in Journal of Clinical Oncology, to patients seen in their own clinical practice.
PMID:33275489 | DOI:10.1200/JCO.20.02720
View details for PubMedID 33275489
-
More
-
Blocking Y-Box Binding Protein-1 through Simultaneous Targeting of PI3K and MAPK in Triple Negative Breast Cancers Cancers
Tiwari A, Iida M, Kosnopfel C, Abbariki M, Menegakis A, Fehrenbacher B, Maier J, Schaller M, Brucker SY, Wheeler DL, Harari PM, Rothbauer U, Schittek B, Zips D, Toulany M
2020 Sep 29;12(10):2795. doi: 10.3390/cancers12102795.
-
More
The multifunctional protein Y-box binding protein-1 (YB-1) regulates all the so far described cancer hallmarks including cell proliferation and survival. The MAPK/ERK and PI3K/Akt pathways are also the major pathways involved in cell growth, proliferation, and survival, and are the frequently hyperactivated pathways in human cancers. A gain of function mutation in KRAS mainly leads to the constitutive activation of the MAPK pathway, while the activation of the PI3K/Akt pathway occurs either through the loss of PTEN or a gain of function mutation of the catalytic subunit alpha of PI3K (PIK3CA). In this study, we investigated the underlying signaling pathway involved in YB-1 phosphorylation at serine 102 (S102) in KRAS(G13D)-mutated triple-negative breast cancer (TNBC) MDA-MB-231 cells versus PIK3CA(H1047R)/PTEN(E307K) mutated TNBC MDA-MB-453 cells. Our data demonstrate that S102 phosphorylation of YB-1 in KRAS-mutated cells is mainly dependent on the MAPK/ERK pathway, while in PIK3CA/PTEN-mutated cells, YB-1 S102 phosphorylation is entirely dependent on the PI3K/Akt pathway. Independent of the individual dominant pathway regulating YB-1 phosphorylation, dual targeting of MEK and PI3K efficiently inhibited YB-1 phosphorylation and blocked cell proliferation. This represents functional crosstalk between the two pathways. Our data obtained from the experiments, applying pharmacological inhibitors and genetic approaches, shows that YB-1 is a key player in cell proliferation, clonogenic activity, and tumor growth of TNBC cells through the MAPK and PI3K pathways. Therefore, dual inhibition of these two pathways or single targeting of YB-1 may be an effective strategy to treat TNBC.
PMID:33003386 | PMC:PMC7601769 | DOI:10.3390/cancers12102795
View details for PubMedID 33003386
-
More
-
Life Beyond COVID: Pay Attention to Viruses International journal of radiation oncology, biology, physics
Harari PM, Lambert PF
2020 Oct 1;108(2):348-350. doi: 10.1016/j.ijrobp.2020.07.001.
-
More
PMID:32890509 | PMC:PMC7462873 | DOI:10.1016/j.ijrobp.2020.07.001
View details for PubMedID 32890509
-
More
-
Practice recommendations for risk-adapted head and neck cancer radiotherapy during the COVID-19 pandemic: An ASTRO-ESTRO consensus statement Radiotherapy and oncology : journal of the European Society for Therapeutic Radiology and Oncology
Thomson DJ, Palma D, Guckenberger M, Balermpas P, Beitler JJ, Blanchard P, Brizel D, Budach W, Caudell J, Corry J, Corvo R, Evans M, Garden AS, Giralt J, Gregoire V, Harari PM, Harrington K, Hitchcock YJ, Johansen J, Kaanders J, Koyfman S, Langendijk JA, Le Q, Lee N, Margalit D, Mierzwa M, Porceddu S, Soong YL, Sun Y, Thariat J, Waldron J, Yom SS
2020 Oct;151:314-321. doi: 10.1016/j.radonc.2020.04.019. Epub 2020 Jul 27.
-
More
PURPOSE: Because of the unprecedented disruption of health care services caused by the COVID-19 pandemic, the American Society of Radiation Oncology (ASTRO) and the European Society for Radiotherapy and Oncology (ESTRO) identified an urgent need to issue practice recommendations for radiation oncologists treating head and neck cancer (HNC) in a time of limited resources and heightened risk for patients and staff.
METHODS AND MATERIALS: A panel of international experts from ASTRO, ESTRO, and select Asia-Pacific countries completed a modified rapid Delphi process. Topics and questions were presented to the group, and subsequent questions were developed from iterative feedback. Each survey was open online for 24 hours, and successive rounds started within 24 hours of the previous round. The chosen cutoffs for strong agreement (≥80%) and agreement (≥66%) were extrapolated from the RAND methodology. Two pandemic scenarios, early (risk mitigation) and late (severely reduced radiation therapy resources), were evaluated. The panel developed treatment recommendations for 5 HNC cases.
RESULTS: In total, 29 of 31 of those invited (94%) accepted, and after a replacement 30 of 30 completed all 3 surveys (100% response rate). There was agreement or strong agreement across a number of practice areas, including treatment prioritization, whether to delay initiation or interrupt radiation therapy for intercurrent SARS-CoV-2 infection, approaches to treatment (radiation dose-fractionation schedules and use of chemotherapy in each pandemic scenario), management of surgical cases in event of operating room closures, and recommended adjustments to outpatient clinic appointments and supportive care.
CONCLUSIONS: This urgent practice recommendation was issued in the knowledge of the very difficult circumstances in which our patients find themselves at present, navigating strained health care systems functioning with limited resources and at heightened risk to their health during the COVID-19 pandemic. The aim of this consensus statement is to ensure high-quality HNC treatments continue, to save lives and for symptomatic benefit.
PMID:32730830 | PMC:PMC7384409 | DOI:10.1016/j.radonc.2020.04.019
View details for PubMedID 32730830
-
More
-
The Promise of Combining Radiation Therapy With Immunotherapy International journal of radiation oncology, biology, physics
Jagodinsky JC, Harari PM, Morris ZS
2020 Sep 1;108(1):6-16. doi: 10.1016/j.ijrobp.2020.04.023. Epub 2020 Apr 23.
-
More
The development of immunotherapy in oncology builds upon many years of scientific investigation into the cellular mechanics underlying interactions between tumor cells and immune cell populations. The past decade has brought an accelerating pace to the clinical investigation of new immunotherapy agents, particularly in the setting of metastatic disease. The integration of immunotherapy into phase 3 clinical trial design has lagged in settings of advanced locoregional disease, where combination with radiation therapy may be critical. Yet, such may be the settings where immunotherapies have their greatest potential to affect patient survival and achieve curative outcomes. In this review, we discuss the interaction of radiation with the immune system and the potential to augment antitumor immunity through combined-modality approaches that integrate radiation and immunotherapies. The dynamics of cellular and tumor response to radiation offer unique opportunities for beneficial interplay with immunotherapy that may go unrecognized with conventional screening and monotherapy clinical testing of novel pharmaceutical agents. Using immune checkpoint blockade as a primary example, we discuss recent preclinical and clinical studies that illustrate the potential synergy of such therapies in combination with radiation, and we highlight the potential clinical value of such interactions. For various immunotherapy agents, their greatest clinical effect may rest in combination with radiation, and efforts to facilitate systematic investigation of this approach are highly warranted.
PMID:32335187 | PMC:PMC7442714 | DOI:10.1016/j.ijrobp.2020.04.023
View details for PubMedID 32335187
-
More
-
Practice Recommendations for Risk-Adapted Head and Neck Cancer Radiation Therapy During the COVID-19 Pandemic: An ASTRO-ESTRO Consensus Statement International journal of radiation oncology, biology, physics
Thomson DJ, Palma D, Guckenberger M, Balermpas P, Beitler JJ, Blanchard P, Brizel D, Budach W, Caudell J, Corry J, Corvo R, Evans M, Garden AS, Giralt J, Gregoire V, Harari PM, Harrington K, Hitchcock YJ, Johansen J, Kaanders J, Koyfman S, Langendijk JA, Le Q, Lee N, Margalit D, Mierzwa M, Porceddu S, Soong YL, Sun Y, Thariat J, Waldron J, Yom SS
2020 Jul 15;107(4):618-627. doi: 10.1016/j.ijrobp.2020.04.016. Epub 2020 Apr 14.
-
More
PURPOSE: Because of the unprecedented disruption of health care services caused by the COVID-19 pandemic, the American Society of Radiation Oncology (ASTRO) and the European Society for Radiotherapy and Oncology (ESTRO) identified an urgent need to issue practice recommendations for radiation oncologists treating head and neck cancer (HNC) in a time of limited resources and heightened risk for patients and staff.
METHODS AND MATERIALS: A panel of international experts from ASTRO, ESTRO, and select Asia-Pacific countries completed a modified rapid Delphi process. Topics and questions were presented to the group, and subsequent questions were developed from iterative feedback. Each survey was open online for 24 hours, and successive rounds started within 24 hours of the previous round. The chosen cutoffs for strong agreement (≥80%) and agreement (≥66%) were extrapolated from the RAND methodology. Two pandemic scenarios, early (risk mitigation) and late (severely reduced radiation therapy resources), were evaluated. The panel developed treatment recommendations for 5 HNC cases.
RESULTS: In total, 29 of 31 of those invited (94%) accepted, and after a replacement 30 of 30 completed all 3 surveys (100% response rate). There was agreement or strong agreement across a number of practice areas, including treatment prioritization, whether to delay initiation or interrupt radiation therapy for intercurrent SARS-CoV-2 infection, approaches to treatment (radiation dose-fractionation schedules and use of chemotherapy in each pandemic scenario), management of surgical cases in event of operating room closures, and recommended adjustments to outpatient clinic appointments and supportive care.
CONCLUSIONS: This urgent practice recommendation was issued in the knowledge of the very difficult circumstances in which our patients find themselves at present, navigating strained health care systems functioning with limited resources and at heightened risk to their health during the COVID-19 pandemic. The aim of this consensus statement is to ensure high-quality HNC treatments continue, to save lives and for symptomatic benefit.
PMID:32302681 | PMC:PMC7194855 | DOI:10.1016/j.ijrobp.2020.04.016
View details for PubMedID 32302681
-
More
-
Fibroblast Growth Factor Receptors as Targets for Radiosensitization in Head and Neck Squamous Cell Carcinomas International journal of radiation oncology, biology, physics
Fisher MM, SenthilKumar G, Hu R, Goldstein S, Ong IM, Miller MC, Brennan SR, Kaushik S, Abel L, Nickel KP, Iyer G, Harari PM, Kimple RJ, Baschnagel AM
2020 Jul 15;107(4):793-803. doi: 10.1016/j.ijrobp.2020.03.040. Epub 2020 Apr 13.
-
More
PURPOSE: We examined the capacity of the pan-fibroblast growth factor receptor (FGFR) inhibitor AZD4547 to augment radiation response across a panel of head and neck squamous cell carcinoma (HNSCC) cell lines and xenografts.
METHODS AND MATERIALS: FGFR1, FGFR2, and FGFR3 RNA in situ hybridization expression was assessed in a cohort of HNSCC patient samples, cell lines, and patient-derived xenografts (PDXs). In vitro effects of AZD4547 and radiation on cell survival, FGFR signaling, apoptosis, autophagy, cell cycle, and DNA damage repair were evaluated. Reverse phase protein array was used to identify differentially phosphorylated proteins in cells treated with AZD4547. In vivo tumor responses were evaluated in cell lines and PDX models.
RESULTS: FGFR1, FGFR2, and FGFR3 RNA in situ hybridization were expressed in 41%, 81%, and 89% of 107 oropharynx patient samples. Sensitivity to AZD4547 did not directly correlate with FGFR protein or RNA expression. In sensitive cell lines, AZD4547 inhibited p-MAPK in a time-dependent manner. Significant radiosensitization with AZD4547 was observed in cell lines that were sensitive to AZD4547. The mechanism underlying these effects appears to be multifactorial, involving inhibition of the MTOR pathway and subsequent enhancement of autophagy and activation of apoptotic pathways. Significant tumor growth delay was observed when AZD4547 was combined with radiation compared with radiation or drug alone in an FGFR-expressing HNSCC cell line xenograft and PDX.
CONCLUSIONS: These findings suggest that AZD4547 can augment the response of radiation in FGFR-expressing HNSCC in vivo model systems. FGFR1 and FGFR2 may prove worthy targets for radiosensitization in HNSCC clinical investigations.
PMID:32298810 | PMC:PMC7321889 | DOI:10.1016/j.ijrobp.2020.03.040
View details for PubMedID 32298810
-
More
-
Emphasize Treatment of Known Disease Rather Than Past Footprints International journal of radiation oncology, biology, physics
Witek ME, Harari PM
2020 Apr 1;106(5):904. doi: 10.1016/j.ijrobp.2019.09.023.
-
Clinical outcomes for larynx patients with cancer treated with refinement of high-dose radiation treatment volumes Head & neck
Burr AR, Harari PM, Haasl AM, Wieland AM, Bruce JY, Kimple RJ, Hartig GK, McCulloch TM, Witek ME
2020 Aug;42(8):1874-1881. doi: 10.1002/hed.26098. Epub 2020 Feb 14.
-
More
BACKGROUND: To evaluate disease control, toxicities, and dose to dysphagia/aspiration risk structures (DARS) using a direct gross tumor volume (GTV70Gy ) to planning target volume expansion (dPTV70Gy ) for patients with squamous cell carcinoma of the larynx (LSCC).
METHODS: A retrospective review was performed on patients with LSCC treated between 2003 and 2018. Clinical outcomes, toxicities, and dosimetric data were analyzed.
RESULTS: Seventy-three patients were identified. Overall survival at 5-years was 57.8%. Five-year local and regional control was 79.8% and 88.2%, respectively. Distant metastatic-only failure was 2.7%. Eighty percent of failures were 95% contained within the dPTV70Gy . Mean dose and the volume of DARS receiving 70 Gy was significantly lower for dPTV70Gy compared to a consensus-defined PTV70Gy .
DISCUSSION: Judicious reduction in high-dose target volumes can preserve high tumor control rates while reducing dose to normal surrounding structures underscoring the potential benefit of this approach in enabling local therapy intensification to improve locoregional control.
PMID:32057151 | PMC:PMC7369226 | DOI:10.1002/hed.26098
View details for PubMedID 32057151
-
More
-
Human Tumor-Lymphatic Microfluidic Model Reveals Differential Conditioning of Lymphatic Vessels by Breast Cancer Cells Advanced healthcare materials
Ayuso JM, Gong MM, Skala MC, Harari PM, Beebe DJ
2020 Feb;9(3):e1900925. doi: 10.1002/adhm.201900925. Epub 2020 Jan 1.
-
More
Breast tumor progression is a complex process involving intricate crosstalk between the primary tumor and its microenvironment. In the context of breast tumor-lymphatic interactions, it is unclear how breast cancer cells alter the gene expression of lymphatic endothelial cells and how these transcriptional changes potentiate lymphatic dysfunction. Thus, there is a need for in vitro lymphatic vessel models to study these interactions. In this work, a tumor-lymphatic microfluidic model is developed to study the differential conditioning of lymphatic vessels by estrogen receptor-positive (i.e., MCF7) and triple-negative (i.e., MDA-MB-231) breast cancer cells. The model consists of a lymphatic endothelial vessel cultured adjacently to either MCF7 or MDA-MB-231 cells. Quantitative transcriptional analysis reveals expression changes in genes related to vessel growth, permeability, metabolism, hypoxia, and apoptosis in lymphatic endothelial cells cocultured with breast cancer cells. Interestingly, these changes are different in the MCF7-lymphatic cocultures as compared to the 231-lymphatic cocultures. Importantly, these changes in gene expression correlate to functional responses, such as endothelial barrier dysfunction. These results collectively demonstrate the utility of this model for studying breast tumor-lymphatic crosstalk for multiple breast cancer subtypes.
PMID:31894641 | PMC:PMC7004876 | DOI:10.1002/adhm.201900925
View details for PubMedID 31894641
-
More
-
Clinical Implications of Scleroderma in the Decision for Radiotherapy-Based Larynx Preservation JAMA otolaryngology-- head & neck surgery
Rydzewski NR, Harari PM, Hartig GK, Francis D, Witek ME
2020 Mar 1;146(3):308-309. doi: 10.1001/jamaoto.2019.3911.
-
Human organotypic lymphatic vessel model elucidates microenvironment-dependent signaling and barrier function Biomaterials
Gong MM, Lugo-Cintron KM, White BR, Kerr SC, Harari PM, Beebe DJ
2019 Sep;214:119225. doi: 10.1016/j.biomaterials.2019.119225. Epub 2019 May 25.
-
More
The lymphatic system is an active player in the pathogenesis of several human diseases, including lymphedema and cancer. Relevant models are needed to advance our understanding of lymphatic biology in disease progression to improve therapy and patient outcomes. Currently, there are few 3D in vitro lymphatic models that can recapitulate the physiological structure, function, and interactions of lymphatic vessels in normal and diseased microenvironments. Here, we developed a 3D microscale lymphatic vessel (μLYMPH) system for generating human lymphatic vessels with physiological tubular structure and function. Consistent with characteristics of lymphatic vessels in vivo, the endothelium of cultured vessels was leaky with an average permeability of 1.38 × 10-5 ± 0.29 × 10-5 cm/s as compared to 0.68 × 10-5 ± 0.13 × 10-5 cm/s for blood vessels. This leakiness also resulted in higher uptake of solute by the lymphatic vessels under interstitial flow, demonstrating recapitulation of their natural draining function. The vessels secreted appropriate growth factors and inflammatory mediators. Our system identified the follistatin/activin axis as a novel pathway in lymphatic vessel maintenance and inflammation. Moreover, the μLYMPH system provided a platform for examining crosstalk between lymphatic vessels and tumor microenvironmental components, such as breast cancer-associated fibroblasts (CAFs). In co-culture with CAFs, vessel barrier function was significantly impaired by CAF-secreted IL-6, a possible pro-metastatic mechanism of lymphatic metastasis. Targeted blocking of the IL-6/IL-6R signaling pathway with an IL-6 neutralizing antibody fully rescued the vessels, demonstrating the potential of our system for screening therapeutic targets. These results collectively demonstrate the μLYMPH system as a powerful model for advancing lymphatic biology in health and disease.
PMID:31154151 | PMC:PMC7032699 | DOI:10.1016/j.biomaterials.2019.119225
View details for PubMedID 31154151
-
More
-
Reducing radiotherapy target volume expansion for patients with HPV-associated oropharyngeal cancer Oral oncology
Burr AR, Harari PM, Ko HC, Bruce JY, Kimple RJ, Witek ME
2019 May;92:52-56. doi: 10.1016/j.oraloncology.2019.03.013. Epub 2019 Mar 22.
-
More
PURPOSE: To evaluate clinical outcomes and patterns of failure using a direct gross tumor volume to planning target volume expansion in patients with p16-positive oropharyngeal squamous cell carcinoma.
METHODS AND MATERIALS: We performed a retrospective review of patients with p16-positive oropharyngeal squamous cell carcinomas treated between 2002 and 2017 with primary radiotherapy with or without concurrent systemic therapy. Patient and disease characteristics associated with disease control and clinical outcomes were analyzed by Cox proportional hazards regression and Kaplan-Meier analyses. Imaging at the time of first failure was used to categorize failure patterns.
RESULTS: We identified 134 patients with a median follow-up of 56.2 months (range 8.2-160.2 months). Local and regional control at 5 years was 91.5% (95% CI: 86.8-96.4%), and 90.8% (95% CI: 85.6-96.2%), respectively. Of the 14 locoregional failures, there were 10 in-field (Type A), 3 marginal (Type B), and 1 geographic (Type E). Age >70 years (HR 5.42; 95% CI: 1.87-15.68) and T4 versus T1-3 (HR 4.09; 95% CI: 1.01-2.65) were associated with increased rates of locoregional failure on multivariate analysis. The rate of gastrostomy tube retention at one year was 6.0% (range 2.8-12.7%).
CONCLUSIONS: Management of patients with p16-positive oropharyngeal squamous cell carcinoma using definitive radiotherapy and a high-dose planning target volume created without a gross tumor volume to clinical tumor volume expansion resulted in high locoregional control with the vast majority of failures occurring within the high-dose field. These data warrant prospective evaluation of this technique as a therapy de-intensification approach.
PMID:31010623 | PMC:PMC7062456 | DOI:10.1016/j.oraloncology.2019.03.013
View details for PubMedID 31010623
-
More
-
Lessons Learned From Hurricane Maria in Puerto Rico: Practical Measures to Mitigate the Impact of a Catastrophic Natural Disaster on Radiation Oncology Patients Practical radiation oncology
Gay HA, Santiago R, Gil B, Remedios C, Montes PJ, López-Araujo J, Chévere CM, Imbert WS, White J, Arthur DW, Horton JK, Jagsi R, Rabinovich R, Beriwal S, Viswanathan A, Erickson BA, Rengan R, Palma D, Loo BW, Kavanaugh JA, Bradley J, Yom SS, Harari PM, Burnett OL
2019 Sep-Oct;9(5):305-321. doi: 10.1016/j.prro.2019.03.007. Epub 2019 Apr 15.
-
More
PURPOSE: Although the wind, rain, and flooding of Hurricane Maria in Puerto Rico abated shortly after its landfall on September 20, 2017, the disruption of the electrical, communications, transportation, and medical infrastructure of the island was unprecedented in scope and caused lasting harm for many months afterward. A compilation of recommendations from radiation oncologists who were in Puerto Rico during the disaster, and from a panel of American Society for Radiation Oncology (ASTRO) cancer experts was created.
METHODS AND MATERIALS: Radiation oncologists throughout Puerto Rico collaborated and improvised to continue treating patients in the immediate aftermath of the storm and as routine clinical operations were restored gradually. Empirical lessons from the experience of radiation therapy administration in this profoundly altered context of limited resources, impaired communication, and inadequate transportation were organized into a recommended template, applicable to any radiation oncology practice. ASTRO disease-site experts provided evidence-guidelines for mitigating the impact of a 2- to 3-week interruption in radiation therapy.
RESULTS: Practical measures to mitigate the medical impact of a disaster are summarized within the framework of "Prepare, Communicate, Operate, Compensate." Specific measures include the development of an emergency operations plan tailored to specific circumstances, prospective coordination with other radiation oncology clinics before a disaster, ongoing communications with emergency management organizations, and routine practice of alternate methods to disseminate information among providers and patients.
CONCLUSIONS: These recommendations serve as a starting point to assist any radiation oncology practice in becoming more resiliently prepared for a local or regional disruption from any cause. Disease-site experts provide evidence-based guidelines on how to mitigate the impact of a 2- to 3-week interruption in radiation therapy for lung, head and neck, uterine cervix, breast, and prostate cancers through altered fractionation or dose escalation.
PMID:30999000 | DOI:10.1016/j.prro.2019.03.007
View details for PubMedID 30999000
-
More
-
Auriculotemporal Nerve Involvement in Parotid Bed Malignancy The Annals of otology, rhinology, and laryngology
Thompson JD, Avey GD, Wieland AM, Harari PM, Glazer TA, McCulloch TM, Hartig GK
2019 Jul;128(7):647-653. doi: 10.1177/0003489419837574. Epub 2019 Mar 20.
-
More
OBJECTIVE: To identify and evaluate patients with parotid bed malignancy demonstrating radiographic findings of auriculotemporal (AT) nerve involvement.
METHODS: A retrospective review of patients with parotid bed malignancy was performed to identify patients with imaging findings of AT nerve involvement and record associated clinical findings, symptoms, and pathology information. Independent, blinded review of radiographic images by a senior neuroradiologist was performed to identify imaging characteristics and categorize patients into highly likely or possible involvement groups.
RESULTS: Of 547 patients identified with parotid bed malignancy, 23 patients exhibited radiographic findings suggestive of AT nerve involvement. Thirteen patients met criteria for highly likely involvement, and 10 patients met criteria for possible involvement. Cutaneous malignancy with metastasis to the parotid bed accounted for 11 of 23 patients, and the most common histology was squamous cell carcinoma (9 patients). Primary parotid malignancy accounted for 12 of 23 patients, and the most common histology was salivary ductal carcinoma (3 patients). All 13 highly likely patients reported periauricular pain, and 11 of 13 demonstrated facial weakness. Features suggesting advanced disease included radiographic findings of intracranial involvement (10/23 patients), nonsurgical primary treatment (13/23 patients), and positive margins on pathology report (7/10 patients).
CONCLUSION: AT nerve involvement is an uncommon but important phenomenon that often occurs in the setting of advanced disease and is commonly associated with periauricular pain and coexisting facial weakness. Awareness of the associated clinical features and imaging patterns can allow for appropriate identification of this pattern of spread and help to optimize treatment planning.
PMID:30894024 | DOI:10.1177/0003489419837574
View details for PubMedID 30894024
-
More
-
Personalized Treatment for Lacrimal Sac Adenoid Cystic Carcinoma: Case Report and Literature Review Practical radiation oncology
Bowen RC, Ko HC, Avey GD, Hartig GK, Hu R, Harari PM, Lucarelli MJ
2019 May;9(3):136-141. doi: 10.1016/j.prro.2019.01.011. Epub 2019 Feb 4.
-
A Multi-Institutional Experience of MR-Guided Liver Stereotactic Body Radiation Therapy Advances in radiation oncology
Rosenberg SA, Henke LE, Shaverdian N, Mittauer K, Wojcieszynski AP, Hullett CR, Kamrava M, Lamb J, Cao M, Green OL, Kashani R, Paliwal B, Bayouth J, Harari PM, Olsen JR, Lee P, Parikh PJ, Bassetti M
2018 Aug 23;4(1):142-149. doi: 10.1016/j.adro.2018.08.005. eCollection 2019 Jan-Mar.
-
More
PURPOSE: Daily magnetic resonance (MR)-guided radiation has the potential to improve stereotactic body radiation therapy (SBRT) for tumors of the liver. Magnetic resonance imaging (MRI) introduces unique variables that are untested clinically: electron return effect, MRI geometric distortion, MRI to radiation therapy isocenter uncertainty, multileaf collimator position error, and uncertainties with voxel size and tracking. All could lead to increased toxicity and/or local recurrences with SBRT. In this multi-institutional study, we hypothesized that direct visualization provided by MR guidance could allow the use of small treatment volumes to spare normal tissues while maintaining clinical outcomes despite the aforementioned uncertainties in MR-guided treatment.
METHODS AND MATERIALS: Patients with primary liver tumors or metastatic lesions treated with MR-guided liver SBRT were reviewed at 3 institutions. Toxicity was assessed using National Cancer Institute Common Terminology Criteria for Adverse Events Version 4. Freedom from local progression (FFLP) and overall survival were analyzed with the Kaplan-Meier method and χ2 test.
RESULTS: The study population consisted of 26 patients: 6 hepatocellular carcinomas, 2 cholangiocarcinomas, and 18 metastatic liver lesions (44% colorectal metastasis). The median follow-up was 21.2 months. The median dose delivered was 50 Gy at 10 Gy/fraction. No grade 4 or greater gastrointestinal toxicities were observed after treatment. The 1-year and 2-year overall survival in this cohort is 69% and 60%, respectively. At the median follow-up, FFLP for this cohort was 80.4%. FFLP for patients with hepatocellular carcinomas, colorectal metastasis, and all other lesions were 100%, 75%, and 83%, respectively.
CONCLUSIONS: This study describes the first clinical outcomes of MR-guided liver SBRT. Treatment was well tolerated by patients with excellent local control. This study lays the foundation for future dose escalation and adaptive treatment for liver-based primary malignancies and/or metastatic disease.
PMID:30706022 | PMC:PMC6349638 | DOI:10.1016/j.adro.2018.08.005
View details for PubMedID 30706022
-
More
-
Is HPV-Associated Oropharyngeal Cancer Becoming More Common in Older Patients? Laryngoscope investigative otolaryngology
Thompson JD, Harari PM, Hartig GK
2018 Oct 15;3(6):446-449. doi: 10.1002/lio2.181. eCollection 2018 Dec.
-
More
OBJECTIVE: To evaluate changing age demographics over a 15-year period for patients with HPV-associated oropharyngeal squamous cell carcinoma (OPSCC).
STUDY DESIGN: Retrospective review of patients identified with p16-positive OPSCC at our institution over a 15-year timeframe. Materials/Methods: p16-positive immunohistochemistry was used as a surrogate for HPV-associated OPSCC. Patients were categorized according to year of diagnosis (2002-2010 versus 2011-2016). Mean age and proportion of patients over age 65 were statistically evaluated and compared.
RESULTS: From 2002 to 2010, 100 patients were identified with p16-positive OPSCC, mean age at diagnosis was 55.2, and the proportion of patients over 65 was 10.0%. From 2011 to 2016, 188 patients were identified with p16-positive OPSCC, mean age was 58.5, and the proportion of patients over 65 was 19.6%. Both the mean age difference and the difference in proportion of patients over 65 were statistically significant (P = .001 and P = .034, respectively).
CONCLUSION: The mean age at diagnosis and proportion of patients over 65 has increased over the past 15 years at our institution. This data suggests that HPV-associated OPSCC is being diagnosed more frequently in older persons and that the age demographic may be shifting. Confirmation of this trend with larger patient numbers on a national level will be valuable. This study highlights the importance of maintaining a high clinical suspicion for HPV-associated OPSCC regardless of patient age.
LEVEL OF EVIDENCE: 4.
PMID:30599028 | PMC:PMC6302704 | DOI:10.1002/lio2.181
View details for PubMedID 30599028
-
More
-
Radiotherapy plus cetuximab or cisplatin in human papillomavirus-positive oropharyngeal cancer (NRG Oncology RTOG 1016): a randomised, multicentre, non-inferiority trial Lancet (London, England)
Gillison ML, Trotti AM, Harris J, Eisbruch A, Harari PM, Adelstein DJ, Jordan CK, Zhao W, Sturgis EM, Burtness B, Ridge JA, Ringash J, Galvin J, Yao M, Koyfman SA, Blakaj DM, Razaq MA, Colevas AD, Beitler JJ, Jones CU, Dunlap NE, Seaward SA, Spencer S, Galloway TJ, Phan J, Dignam JJ, Le QT
2019 Jan 5;393(10166):40-50. doi: 10.1016/S0140-6736(18)32779-X. Epub 2018 Nov 15.
-
More
BACKGROUND: Patients with human papillomavirus (HPV)-positive oropharyngeal squamous cell carcinoma have high survival when treated with radiotherapy plus cisplatin. Whether replacement of cisplatin with cetuximab-an antibody against the epidermal growth factor receptor-can preserve high survival and reduce treatment toxicity is unknown. We investigated whether cetuximab would maintain a high proportion of patient survival and reduce acute and late toxicity.
METHODS: RTOG 1016 was a randomised, multicentre, non-inferiority trial at 182 health-care centres in the USA and Canada. Eligibility criteria included histologically confirmed HPV-positive oropharyngeal carcinoma; American Joint Committee on Cancer 7th edition clinical categories T1-T2, N2a-N3 M0 or T3-T4, N0-N3 M0; Zubrod performance status 0 or 1; age at least 18 years; and adequate bone marrow, hepatic, and renal function. We randomly assigned patients (1:1) to receive either radiotherapy plus cetuximab or radiotherapy plus cisplatin. Randomisation was balanced by using randomly permuted blocks, and patients were stratified by T category (T1-T2 vs T3-T4), N category (N0-N2a vs N2b-N3), Zubrod performance status (0 vs 1), and tobacco smoking history (≤10 pack-years vs >10 pack-years). Patients were assigned to receive either intravenous cetuximab at a loading dose of 400 mg/m2 5-7 days before radiotherapy initiation, followed by cetuximab 250 mg/m2 weekly for seven doses (total 2150 mg/m2), or cisplatin 100 mg/m2 on days 1 and 22 of radiotherapy (total 200 mg/m2). All patients received accelerated intensity-modulated radiotherapy delivered at 70 Gy in 35 fractions over 6 weeks at six fractions per week (with two fractions given on one day, at least 6 h apart). The primary endpoint was overall survival, defined as time from randomisation to death from any cause, with non-inferiority margin 1·45. Primary analysis was based on the modified intention-to-treat approach, whereby all patients meeting eligibility criteria are included. This study is registered with ClinicalTrials.gov, number NCT01302834.
FINDINGS: Between June 9, 2011, and July 31, 2014, 987 patients were enrolled, of whom 849 were randomly assigned to receive radiotherapy plus cetuximab (n=425) or radiotherapy plus cisplatin (n=424). 399 patients assigned to receive cetuximab and 406 patients assigned to receive cisplatin were subsequently eligible. After median follow-up duration of 4·5 years, radiotherapy plus cetuximab did not meet the non-inferiority criteria for overall survival (hazard ratio [HR] 1·45, one-sided 95% upper CI 1·94; p=0·5056 for non-inferiority; one-sided log-rank p=0·0163). Estimated 5-year overall survival was 77·9% (95% CI 73·4-82·5) in the cetuximab group versus 84·6% (80·6-88·6) in the cisplatin group. Progression-free survival was significantly lower in the cetuximab group compared with the cisplatin group (HR 1·72, 95% CI 1·29-2·29; p=0·0002; 5-year progression-free survival 67·3%, 95% CI 62·4-72·2 vs 78·4%, 73·8-83·0), and locoregional failure was significantly higher in the cetuximab group compared with the cisplatin group (HR 2·05, 95% CI 1·35-3·10; 5-year proportions 17·3%, 95% CI 13·7-21·4 vs 9·9%, 6·9-13·6). Proportions of acute moderate to severe toxicity (77·4%, 95% CI 73·0-81·5 vs 81·7%, 77·5-85·3; p=0·1586) and late moderate to severe toxicity (16·5%, 95% CI 12·9-20·7 vs 20·4%, 16·4-24·8; p=0·1904) were similar between the cetuximab and cisplatin groups.
INTERPRETATION: For patients with HPV-positive oropharyngeal carcinoma, radiotherapy plus cetuximab showed inferior overall survival and progression-free survival compared with radiotherapy plus cisplatin. Radiotherapy plus cisplatin is the standard of care for eligible patients with HPV-positive oropharyngeal carcinoma.
FUNDING: National Cancer Institute USA, Eli Lilly, and The Oral Cancer Foundation.
PMID:30449625 | PMC:PMC6541928 | DOI:10.1016/S0140-6736(18)32779-X
View details for PubMedID 30449625
-
More
Contact Information
Paul Harari, MD
Administrative Assistant: Julie Thomas,600 Highland Avenue, Box 3684 Clinical Science Center
Madison, WI 53792
(608) 263-5009
HN SPORE Contact
HN SPORE Grant Administrator: Shari Piaskowski,,600 Highland Avenue, K4/460 Clinical Science Center
Madison, WI 53792
