I am an associate professor in the Department of Human Oncology at the University of Wisconsin School of Medicine and Public Health, where I treat patients and perform translational research. In my clinical practice, I specialize in treating patients with lung cancer, and other thoracic malignancies. In addition, I have expertise in performing stereotactic body radiotherapy and intracranial stereotactic radiosurgery for the treatment of metastases. I am a member of the UW Multidisciplinary Thoracic Program and work closely with thoracic surgeons, medical oncologists, pulmonologists and radiologists to best meet the needs of my patients. I also serve as the department’s director of clinical trials.
I am actively involved in translational and clinical research. I work closely with basic scientists, physicists and clinicians with the goal of fostering new ideas and translating research findings into the clinic. I have experience studying combinations of radiation and novel radiosensitizers and biomarkers of treatment response in patients and in preclinical models. My current work focuses on improving treatment of non-small cell lung cancer with a focus on brain metastases. I am actively involved at the regional and national level, and I am site PI for multiple national clinical studies here at UW. In addition to my clinical and research activities, I train medical students, medical residents and fellows. This includes mentoring students and residents in the clinic and with research.
Dr. Baschnagel's UW Health ProfileEducation
Resident, William Beaumont Hospital, Radiation Oncology (2014)
Intern, William Beaumont Hospital, (2010)
Research Fellow, National Institutes of Health, Clinical Research (2008)
MD, State University of New York at Buffalo, Medicine (2009)
BS, State University of New York at Buffalo, Biophysical Sciences (2004)
Academic Appointments
Associate Professor, Human Oncology (2020)
Assistant Professor, Human Oncology (2014)
Selected Honors and Awards
Radiation Research Early Career Investigator Travel Award (2019)
American Association of Residents in Radiation Oncology Educator of the Year Award (2018)
UW Paul P. Carbone Young Investigator Award, UW Carbone Cancer Center (2017)
Travel Grant Award, ASTRO Multidisciplinary Head and Neck Cancer Symposium (2014)
First Place, Resident and Fellow Michigan State Medical Society Annual Research Forum (2013)
First Place, Poster Presentation, 43rd Annual Beaumont Research Forum (2013)
First Place, Michigan Radiological Society Radiation Oncology Research Award (2013)
Baccelli Award for Most Outstanding Research, SUNY at Buffalo (2009)
First Honors, Annual Medical Student Research Forum, SUNY at Buffalo (2009)
University at Buffalo School of Medicine Alumni Scholarship (2004–2009)
Outstanding Undergraduate Award, Biophysics Department SUNY at Buffalo (2004)
Academic Excellence Scholarship, SUNY at Buffalo (2000–2004)
Boards, Advisory Committees and Professional Organizations
NRG Oncology Corresponding Principal Investigator for University of Wisconsin School of Medicine and Public Health (2019-pres.)
University of Wisconsin, Department of Human Oncology, Director of Clinical Research (2018-pres.)
Member of Lung Cancer Working Group, Big Ten Cancer Research Consortium (2017–pres.)
Clinical Translational and Basic Science Advisory Committee Member, ASTRO Clinical (2016–pres.)
Quality Control Committee Chair, UWCCC Department of Human Oncology (2016–pres.)
Peer Review Committee Member, UW Hospital and Clinics, (2016–pres.)
Abstract reviewer, ASTRO Annual Meeting Lung Committee Member (2015–pres.)
Protocol Review and Monitoring Committee Member, UWCCC (2014–pres.)
Research Focus
Translational Research, Stereotactic Body Radiotherapy, Intracranial Stereotactic Radiosurgery, Novel Radiosensitizers
Dr. Andrew Baschnagel is a physician who specializes in treating patients with lung, esophageal and other thoracic malignancies. His current translational and clinical research focuses on improving treatment of non-small cell lung cancer, esophageal cancer, head and neck cancer and pancreas cancer. He also serves as the department’s director of clinical trials.
My research objective is to translate novel findings from the laboratory into the clinic with the goal of improving treatment for cancer patients.
Precision medicine in radiation oncology involves individually optimizing and tailoring radiation treatments to improve outcomes and decrease side effects. This can be done by selectively combining molecular targeted drugs with radiation to improve the therapeutic ratio and using biomarkers to predict radiation tumor and normal tissue response. These are areas that my research focuses on.
Translational research in lung cancer
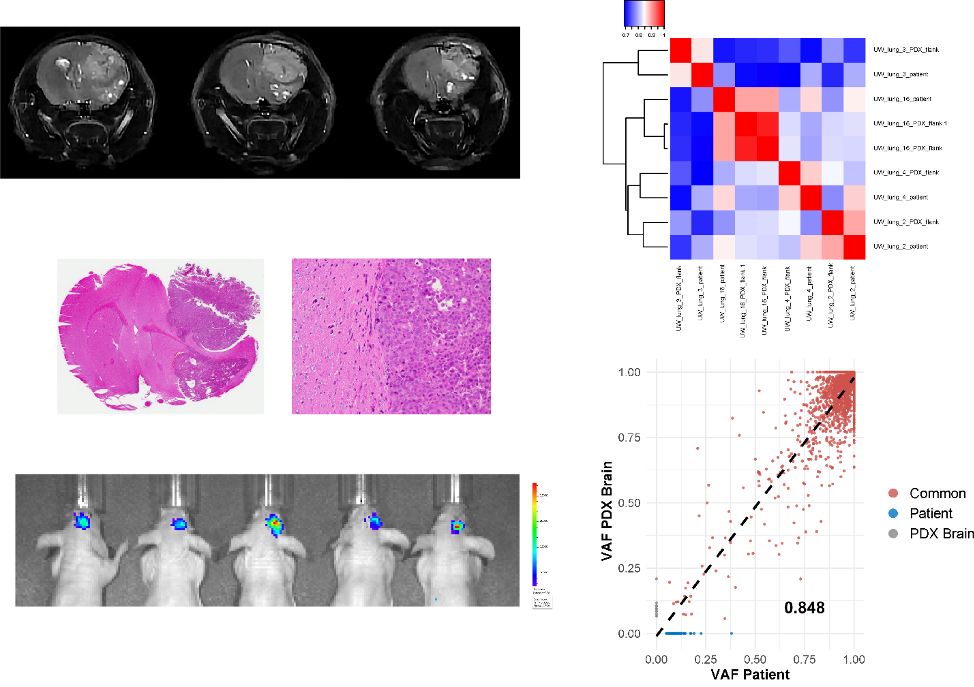
Current efforts are focused on understanding the molecular basis for the development and progression of non-small cell lung cancer (NSCLC) brain metastases as well as strategies to improve the therapeutic ratio of radiation. Brain metastases from NSCLC remain a significant challenge to treat and unfortunately lead to the death of many patients. Understanding the biology of brain metastases may shed some light on how they develop and provide novel targets for therapeutics. We currently have an ongoing project examining the genomics differences between patient NSCLC pulmonary tumors and NSCLC brain metastases. The goal is to determine molecular differences that might drive the development of brain metastases.
One of the major barriers to improving outcomes and developing novel treatments is the lack of robust preclinical brain metastases models. Working with the Kimple Lab, we currently have established patient-derived xenografts from NSCLC brain metastases and are using them to test novel therapeutic agents. Patient-derived xenografts can provide an excellent model system to study preclinical therapies as they allow a better representation of the biology of their human source than cells grown in plastic tissue culture dishes. These models will be used to validate molecular targets identified in our genomics endeavor and will be used to test combinations of drugs and radiation.
Relevant Publications:
- Baschnagel AM, Elnaggar JH, VanBeek HJ, Kromke AC, Skiba JH, Kaushik S, Abel L, Clark PA, Longhurst CA, Nickel KP, Leal TA, Zhao SG, Kimple RJ. ATR inhibitor M6620 (VX-970) enhances the effect of radiation in non-small cell lung cancer brain metastasis patient derived xenografts. Mol Cancer Ther. 2021 Aug 19:molcanther.0305.2021. doi: 10.1158/1535-7163.MCT-21-0305. Online ahead of print. DOI: 10.1158/1535-7163.MCT-21-0305
- SenthilKumar G, Fisher MM, Skiba JH, Miller MC, Brennan SR, Kaushik S, Bradley ST, Longhurst CA, Buehler D, Nickel KP, Iyer G, Kimple RJ, Baschnagel AM. FGFR inhibition enhances sensitivity to radiation in non-small cell lung cancer. Mol Cancer Ther. 2020;19(6):1255-65. PMCID: PMC7272291
- Baschnagel AM, Kaushik S, Durmaz A, Goldstein S, Ong IM, Abel L, Clark PA, Gurel Z, Leal T, Buehler D, Iyer G, Scott JG, Kimple RJ. Development and Characterization of Patient-Derived Xenografts from Non-Small Cell Lung Cancer Brain Metastases. Sci Rep. 2021;11(1):2520. PMCID:PMC7843608
Molecular modulation of radiation response
This translational research area focuses on studying molecular-targeted agents that can enhance the effect of radiation in preclinical models. Ongoing work involves studying radiosensitization in lung cancer brain metastasis models.
Relevant Publications:
- Baschnagel A, Russo A, Burgan WE, Carter D, Beam K, Palmieri D, Steeg PS, Tofilon P, Camphausen K. Vorinostat enhances the radiosensitivity of a breast cancer brain metastatic cell line grown in vitro and as intracranial xenografts. Mol Cancer Ther. 2009;8(6):1589-95. PMCID: PMC3393105
- Baschnagel AM, Galoforo S, Thibodeau BJ, Ahmed S, Nirmal S, Akervall J, Wilson GD. Crizotinib Fails to Enhance the Effect of Radiation in Head and Neck Squamous Cell Carcinoma Xenografts. Anticancer Res. 2015;35(11):5973-82.
- Fisher MM, SenthilKumar G, Hu R, Goldstein S, Ong IM, Miller MC, Brennan SR, Kaushik S, Abel L, Nickel KP, Iyer G, Harari PM, Kimple RJ, Baschnagel AM. Fibroblast Growth Factor Receptors as Targets for Radiosensitization in Head and Neck Squamous Cell Carcinomas. Int J Radiat Oncol Biol Phys. 2020;107(4):793-803. PMCID: PMC7321889
Molecular markers of radiation response and prognosis
Biomarkers that can predict the response to radiation therapy have not made their way into the clinic. Such markers could help clinicians personalize radiation treatment and help monitor disease status. My past work has focused on expression prognostic makers in patients with head and neck cancer. Current efforts are now focused on non-small cell lung cancer.
Relevant Publications:
- Baschnagel AM, Williams L, Hanna A, Chen PY, Krauss DJ, Pruetz BL, Akervall J, Wilson GD. c-Met expression is a marker of poor prognosis in patients with locally advanced head and neck squamous cell carcinoma treated with chemoradiation. Int J Radiat Oncol Biol Phys. 2014 Mar 1;88(3):701-7. PubMed PMID: 24521684.
- Baschnagel AM, Tonlaar N, Eskandari M, Kumar T, Williams L, Hanna A, Pruetz BL, Wilson GD. Combined CD44, c-MET, and EGFR expression in p16-positive and p16-negative head and neck squamous cell carcinomas. J Oral Pathol Med. 2017 Mar;46(3):208-213. PubMed PMID: 27442811.
- Baschnagel AM, Wobb JL, Dilworth JT, Williams L, Eskandari M, Wu D, Pruetz BL, Wilson GD. The association of (18)F-FDG PET and glucose metabolism biomarkers GLUT1 and HK2 in p16 positive and negative head and neck squamous cell carcinomas. Radiother Oncol. 2015 Oct;117(1):118-24. PubMed PMID: 26328941.
- Wilson TJ, Hana A, Recknagel J, Pruetz BL, Baschnagel AM, Wilson GD. Prognostic significance of MTOR expression in HPV positive and negative head and neck cancers treated by chemoradiation. Head Neck. 2020;42(2):153-62.
Brain metastasis research
This active area of research involves improving clinical outcomes of patients with brain metastases with a focus on stereotactic radiotherapy.
Relevant Publications:
- Baschnagel AM, Meyer KD, Chen PY, Krauss DJ, Olson RE, Pieper DR, Maitz AH, Ye H, Grills IS. Tumor volume as a predictor of survival and local control in patients with brain metastases treated with Gamma Knife surgery. J Neurosurg. 2013;119(5):1139-44.
- Johnson MD, Avkshtol V, Baschnagel AM, Meyer K, Hong Y, Grills IS, Chen PY, Maitz A, Olson RE, Pieper DR, Krauss DJ. Surgical Resection of Brain Metastases and the Risk of Meningeal Recurrence in Patients Treated with Stereotactic Radiosurgery. Int J Radiat Oncol Biol Phys. 2016;94(3):537-43.
- Musunuru HB, Witt JS, Francis DM, Yadav P, Bayliss A, Ko HC, Burr A, Bassetti MF, Howard SP, Baschnagel AM. Impact of adjuvant fractionated stereotactic radiotherapy dose on local control of brain metastases. J Neurooncol. 2019;145(2):385-90.
- Enright TL, Witt JS, Burr AR, Yadav P, Leal T, Baschnagel AM. Combined Immunotherapy and Stereotactic Radiotherapy Improves Neurologic Outcomes in Patients with Non-small-cell Lung Cancer Brain Metastases. Clin Lung Cancer. 2020 Nov 10:S1525-7304(20)30324-7. doi: 10.1016/j.cllc.2020.10.014.
Improving the care of patients with thoracic cancer
I have published many clinical radiation oncology studies that have helped improve patient care. These include studies focused on lung cancer and esophageal cancers. I have expertise in analyzing large data sets including data from the National Cancer Database to answer important clinical questions.
Relevant Publications:
- Brower JV, Chen S, Bassetti MF, Yu M, Harari PM, Ritter MA, Baschnagel AM. Radiation Dose Escalation in Esophageal Cancer Revisited: A Contemporary Analysis of the National Cancer Data Base, 2004 to 2012. Int J Radiat Oncol Biol Phys. 2016;96(5):985-93.
- Brower JV, Amini A, Chen S, Hullett CR, Kimple RJ, Wojcieszynski AP, Bassetti M, Witek ME, Yu M, Harari PM, Baschnagel AM. Improved survival with dose-escalated radiotherapy in stage III non-small-cell lung cancer: analysis of the National Cancer Database. Ann Oncol. 2016;27(10):1887-94. doi: 1093/annonc/mdw276
- Witt JS, Jagodinskyb JC, Liuc Y, Yadava P, Kuczmarska-Haasa A, YuM, Maloney JD, Ritter MA, Bassetti MF, Baschnagel AM. Cardiac Toxicity in Operable Esophageal Cancer Patients Treated With or Without Chemoradiation. Am J Clin Oncol. 2019;42(8):662-7. PMCID: PMC6828548 PMCID:PMC6828548
- Käsmann L, Janssen S, Baschnagel AM, Kruser TJ, Harada H, Aktan M, Rades D. Prognostic factors and outcome of reirradiation for locally recurrent small cell lung cancer-a multicenter study. Transl Lung Cancer Res. 2020;9(2):232-8. PMCID: PMC7225148
I specialize in treating patients with thoracic malignancies. I have expertise in intracranial stereotactic radiosurgery and stereotactic body radiotherapy. I am actively involved in the UW Thoracic Disease Oriented Team and lead multiple clinical trials. At UW we offer a variety of promising clinical trials, including national studies, studies using novel molecular agents in combination with radiation, studies utilizing new technologies and studies examining biomarkers. Our ultimate goal is provide the best treatment for our patients.
Clinical expertise:
- External beam radiation therapy including 3D conformal, Intensity Modulated Radiotherapy (IMRT) and Volumetric Modulated Arc Therapy (VMAT)
- Frame-based and frameless intracranial stereotactic radiosurgery
- Stereotactic body radiotherapy: lung, adrenal, liver, spine, bone
- Image guided radiotherapy (IGRT) including cone-beam CT, PET/CT, MRI/CT and
4D image guidance/treatment planning - ViewRay® real time MRI-guided radiotherapy
- Adaptive radiotherapy treatment planning techniques
Clinical Trials
UW studies:
UW17019: Collection of blood and saliva for biomarker analysis of patients undergoing radiotherapy. PI
UW16037: Improving Pulmonary Function Following Radiation Therapy. Co-PI
National Studies open at UW:
-
Genomic and Immune Landscape of Non-Small Cell Lung Cancer Brain Metastases JCO precision oncology
Liu M, Jagodinsky JC, Callahan SC, Minne RL, Johnson DB, Tomlins SA, Iyer G, Baschnagel AM
2025 Jan;9:e2400690. doi: 10.1200/PO-24-00690. Epub 2025 Feb 21.
-
More
PURPOSE: Metastatic spread of non-small cell lung cancer (NSCLC) to the brain is a commonly occurring and challenging clinical problem, often resulting in patient mortality. Systemic therapies including immunotherapy have modest efficacy in treating brain metastases. Moreover, the local immune environment of brain metastases remains poorly described. This study aims to understand the genomic and immune landscape of NSCLC brain metastases.
METHODS: A total of 3,060 patients with NSCLC sequenced with the Strata Select assay on the Strata Oncology Platform were analyzed. Genomic alterations, tumor mutation burden (TMB), PD-L1 expression, and immune gene expression were compared across different tissue sites and histologies and within brain metastases.
RESULTS: A significant increase in TMB was observed in the brain metastasis samples compared with nonbrain metastasis samples. Mutations in TP53, KRAS, and CDKNA2A were more prevalent within the brain metastasis cohort compared with other tissue locations. In addition, PD-L1 expression was significantly decreased within brain metastasis samples compared with other sites. The overall immune landscape within the brain metastasis samples was largely reduced compared with primary lung samples. However, an immune-enriched brain metastasis cohort was identified with higher expressions of PD-L1 and other immune-related genes.
CONCLUSION: The overall TMB is increased within brain metastases compared with primary lung and other metastasis sites and is associated with a markedly diminished overall immune landscape. The identification of an immune-enriched brain metastasis subgroup suggests potential heterogeneity within the brain metastasis patient cohort, which might have implications for the development of targeted therapies.
PMID:39983077 | DOI:10.1200/PO-24-00690
View details for PubMedID 39983077
-
More
-
Erratum to: Gondi V, Deshmukh S, Brown PD, et al. Sustained Preservation of Cognition and Prevention of Patient-Reported Symptoms With Hippocampal Avoidance During Whole-Brain Radiation Therapy for Brain Metastases: Final Results of NRG Oncology CC001. Int J Radiat Oncol Biol Phys. 2023;117:571-580 International journal of radiation oncology, biology, physics
Gondi V, Deshmukh S, Brown PD, Wefel JS, Armstrong TS, Tome WA, Gilbert MR, Konski A, Robinson CG, Bovi JA, Benzinger LS, Roberge D, Kundapur V, Kaufman I, Shah S, Usuki KY, Baschnagel AM, Mehta MP, Kachnic LA
2025 Feb 1;121(2):571-572. doi: 10.1016/j.ijrobp.2024.08.041.
-
Evaluation of a Novel MET-Targeting Camelid-Derived Antibody in Head and Neck Cancer Molecular pharmaceutics
Minne RL, Luo NY, Mork CM, Wopat MR, Esbona K, Javeri S, Nickel KP, Hernandez R, LeBeau AM, Kimple RJ, Baschnagel AM
2024 Dec 2;21(12):6376-6384. doi: 10.1021/acs.molpharmaceut.4c00938. Epub 2024 Nov 8.
-
More
In head and neck squamous cell carcinoma (HNSCC), the mesenchymal epithelial transition (MET) receptor drives cancer growth, proliferation, and metastasis. MET is known to be overexpressed in HNSCC and, therefore, is an appealing therapeutic target. In this study, we evaluated MET expression in patients with HNSCC and investigated the potential imaging application of a novel MET-binding single-domain camelid antibody using positron emission tomography/computed tomography (PET/CT) in a preclinical MET-expressing HNSCC model. Multiplex immunostaining for MET protein performed on a tissue microarray from 203 patients with HNSCC found 86% of patients to have MET expression, with 14% having high expression and 53% having low MET expression. Using The Cancer Genome Atlas (TCGA) database, high MET RNA expression was associated with worse progression-free survival and overall survival in patients with HPV-negative HSNCC. Utilizing flow cytometry and immunofluorescence, our novel camelid antibody fused to a human IgG Fc chain (1E7-Fc) showed high binding affinity and specificity to high MET-expressing Detroit 562 cells but not to low MET-expressing HNSCC cells. The efficacy and biodistribution of [89Zr]Zr-1E7-Fc as a PET imaging agent was then investigated in a MET-expressing head and neck xenograft model. [89Zr]Zr-1E7-Fc rapidly localized and showed high tumor uptake in Detroit 562 xenografts (8.4% ID/g at 72 h postinjection), with rapid clearance from the circulatory system (2.7 tumor-to-blood radioactivity ratio at 72 h postinjection). Our preclinical data suggest that the camelid antibody 1E7-Fc could be a potential theranostic agent for HNSCC. Further investigations are warranted to confirm these findings in patients and to evaluate 1E7-Fc as an imaging agent and platform to deliver radionuclide or drug therapy to MET-driven cancers.
PMID:39513517 | DOI:10.1021/acs.molpharmaceut.4c00938
View details for PubMedID 39513517
-
More
-
GABA(A) Receptor Activation Drives GABARAP-Nix Mediated Autophagy to Radiation-Sensitize Primary and Brain-Metastatic Lung Adenocarcinoma Tumors Cancers
Bhattacharya D, Barrile R, Toukam DK, Gawali VS, Kallay L, Ahmed T, Brown H, Rezvanian S, Karve A, Desai PB, Medvedovic M, Wang K, Ionascu D, Harun N, Vallabhapurapu S, Wang C, Qi X, Baschnagel AM, Kritzer JA, Cook JM, Krummel AP, Sengupta S
2024 Sep 15;16(18):3167. doi: 10.3390/cancers16183167.
-
More
In non-small cell lung cancer (NSCLC) treatment, radiotherapy responses are not durable and toxicity limits therapy. We find that AM-101, a synthetic benzodiazepine activator of GABA(A) receptor, impairs the viability and clonogenicity of both primary and brain-metastatic NSCLC cells. Employing a human-relevant ex vivo 'chip', AM-101 is as efficacious as docetaxel, a chemotherapeutic used with radiotherapy for advanced-stage NSCLC. In vivo, AM-101 potentiates radiation, including conferring a significant survival benefit to mice bearing NSCLC intracranial tumors generated using a patient-derived metastatic line. GABA(A) receptor activation stimulates a selective-autophagic response via the multimerization of GABA(A) receptor-associated protein, GABARAP, the stabilization of mitochondrial receptor Nix, and the utilization of ubiquitin-binding protein p62. A high-affinity peptide disrupting Nix binding to GABARAP inhibits AM-101 cytotoxicity. This supports a model of GABA(A) receptor activation driving a GABARAP-Nix multimerization axis that triggers autophagy. In patients receiving radiotherapy, GABA(A) receptor activation may improve tumor control while allowing radiation dose de-intensification to reduce toxicity.
PMID:39335139 | PMC:PMC11430345 | DOI:10.3390/cancers16183167
View details for PubMedID 39335139
-
More
-
DARES: A Phase II Trial of Durvalumab and Ablative Radiation in Extensive-Stage Small Cell Lung Cancer Clinical lung cancer
Bestvina CM, Hara HL, Karrison T, Bowar B, Chin J, Garassino MC, Pitroda SP, Thawani R, Vokes EE, Gan G, Zhang J, Baschnagel AM, Campbell TC, Chmura S, Juloori A
2024 Dec;25(8):e448-e452. doi: 10.1016/j.cllc.2024.08.004. Epub 2024 Aug 13.
-
More
BACKGROUND: Immunotherapy in combination with chemotherapy is first-line treatment for patients with extensive-stage small-cell lung cancer (ES-SCLC). Growing evidence suggests that radiation, specifically stereotactic body radiation therapy (SBRT), may enhance the immunogenic response as well as cytoreduce tumor burden. The primary objective of the study is to determine the progression free survival for patients with newly diagnosed ES-SCLC treated with combination multisite SBRT and chemo-immunotherapy (carboplatin, etoposide, and durvalumab).
METHODS: This is a multicenter, single arm, phase 2 study. Patients with treatment-naïve, ES-SCLC will be eligible for this study. Patients will receive durvalumab 1500mg IV q3w, carboplatin AUC 5 to 6 mg/mL q3w, and etoposide 80 to 100 mg/m2 on days 1 to 3 q3w for four cycles, followed by durvalumab 1500mg IV q4w until disease progression or unacceptable toxicity. Ablative radiation will be delivered 1 to 4 extracranial sites in 3 or 5 fractions, determined by location, during cycle 2. The primary endpoint is progression-free survival, measured from day 1 of chemoimmunotherapy. Secondary endpoints include grade ≥3 toxicity by CTCAE v5.0 within three months of RT, overall survival, response rate, time to second line systemic therapy, and time to new distant progression.
CONCLUSIONS: Now that immunotherapy is an established part of ES-SCLC management, it is important to further optimize its use and effect. This study will investigate the progression-free survival of combined SBRT and chemo-immunotherapy in patients with ES-SCLC. In addition, the data from this study may further inform the immunogenic role of SBRT with chemo-immunotherapy, as well as identify clinical, biological, or radiomic prognostic features.
PMID:39242330 | DOI:10.1016/j.cllc.2024.08.004
View details for PubMedID 39242330
-
More
-
Genomic and Immune Landscape Comparison of MET Exon 14 Skipping and MET-Amplified Non-small Cell Lung Cancer Clinical lung cancer
Minne RL, Luo NY, Traynor AM, Huang M, DeTullio L, Godden J, Stoppler M, Kimple RJ, Baschnagel AM
2024 Sep;25(6):567-576.e1. doi: 10.1016/j.cllc.2024.05.001. Epub 2024 May 10.
-
More
BACKGROUND: Mutation or amplification of the mesenchymal-epithelial transition (MET) tyrosine kinase receptor causes dysregulation of receptor function and stimulates tumor growth in non-small cell lung cancer (NSCLC) with the most common mutation being MET exon 14 (METex14). We sought to compare the genomic and immune landscape of MET-altered NSCLC with MET wild-type NSCLC.
METHODS: 18,047 NSCLC tumors were sequenced with Tempus xT assay. Tumors were categorized based on MET exon 14 (METex14) mutations; low MET amplification defined as a copy number gain (CNG) 6-9, high MET amplification defined as CNG ≥ 10, and MET other type mutations. Immuno-oncology (IO) biomarkers and the frequency of other somatic gene alterations were compared across MET-altered and MET wild-type groups.
RESULTS: 276 (1.53%) METex14, 138 (0.76%) high METamp, 63 (0.35%) low METamp, 27 (0.15%) MET other, and 17,543 (97%) MET wild-type were identified. Patients with any MET mutation including METex14 were older, while patients with METex14 were more frequently female and nonsmokers. MET gene expression was highest in METamp tumors. PD-L1 positivity rates were higher in MET-altered groups than MET wild-type. METex14 exhibited the lowest tumor mutational burden (TMB) and lowest neoantigen tumor burden (NTB). METamp exhibited the lowest proportion of CD4 T cells and the highest proportion of NK cells. There were significant differences in co-alterations between METamp and METex14.
CONCLUSIONS: METex14 tumors exhibited differences in IO biomarkers and the somatic landscape compared to non-METex14 NSCLC tumors. Variations in immune profiles can affect immunotherapy selection in MET-altered NSCLC and require further exploration.
PMID:38852006 | DOI:10.1016/j.cllc.2024.05.001
View details for PubMedID 38852006
-
More
-
Leveraging radiomics and machine learning to differentiate radiation necrosis from recurrence in patients with brain metastases Journal of neuro-oncology
Basree MM, Li C, Um H, Bui AH, Liu M, Ahmed A, Tiwari P, McMillan AB, Baschnagel AM
2024 Jun;168(2):307-316. doi: 10.1007/s11060-024-04669-4. Epub 2024 Apr 30.
-
More
OBJECTIVE: Radiation necrosis (RN) can be difficult to radiographically discern from tumor progression after stereotactic radiosurgery (SRS). The objective of this study was to investigate the utility of radiomics and machine learning (ML) to differentiate RN from recurrence in patients with brain metastases treated with SRS.
METHODS: Patients with brain metastases treated with SRS who developed either RN or tumor reccurence were retrospectively identified. Image preprocessing and radiomic feature extraction were performed using ANTsPy and PyRadiomics, yielding 105 features from MRI T1-weighted post-contrast (T1c), T2, and fluid-attenuated inversion recovery (FLAIR) images. Univariate analysis assessed significance of individual features. Multivariable analysis employed various classifiers on features identified as most discriminative through feature selection. ML models were evaluated through cross-validation, selecting the best model based on area under the receiver operating characteristic (ROC) curve (AUC). Specificity, sensitivity, and F1 score were computed.
RESULTS: Sixty-six lesions from 55 patients were identified. On univariate analysis, 27 features from the T1c sequence were statistically significant, while no features were significant from the T2 or FLAIR sequences. For clinical variables, only immunotherapy use after SRS was significant. Multivariable analysis of features from the T1c sequence yielded an AUC of 76.2% (standard deviation [SD] ± 12.7%), with specificity and sensitivity of 75.5% (± 13.4%) and 62.3% (± 19.6%) in differentiating radionecrosis from recurrence.
CONCLUSIONS: Radiomics with ML may assist the diagnostic ability of distinguishing RN from tumor recurrence after SRS. Further work is needed to validate this in a larger multi-institutional cohort and prospectively evaluate it's utility in patient care.
PMID:38689115 | DOI:10.1007/s11060-024-04669-4
View details for PubMedID 38689115
-
More
-
Development of an Engineered Single-Domain Antibody for Targeting MET in Non-Small Cell Lung Cancer Bioconjugate chemistry
Luo NY, Minne RL, Gallant JP, Gunaratne GS, West JL, Javeri S, Robertson AJ, Lake EW, Engle JW, Mixdorf JC, Aluicio-Sarduy E, Nickel KP, Hernandez R, Kimple RJ, Baschnagel AM, LeBeau AM
2024 Mar 20;35(3):389-399. doi: 10.1021/acs.bioconjchem.4c00019. Epub 2024 Mar 12.
-
More
The Mesenchymal Epithelial Transition (MET) receptor tyrosine kinase is upregulated or mutated in 5% of non-small-cell lung cancer (NSCLC) patients and overexpressed in multiple other cancers. We sought to develop a novel single-domain camelid antibody with high affinity for MET that could be used to deliver conjugated payloads to MET expressing cancers. From a naïve camelid variable-heavy-heavy (VHH) domain phage display library, we identified a VHH clone termed 1E7 that displayed high affinity for human MET and was cross-reactive with MET across multiple species. When expressed as a bivalent human Fc fusion protein, 1E7-Fc was found to selectively bind to EBC-1 (MET amplified) and UW-Lung 21 (MET exon 14 mutated) cell lines by flow cytometry and immunofluorescence imaging. Next, we investigated the ability of [89Zr]Zr-1E7-Fc to detect MET expression in vivo by PET/CT imaging. [89Zr]Zr-1E7-Fc demonstrated rapid localization and high tumor uptake in both xenografts with a %ID/g of 6.4 and 5.8 for EBC-1 and UW-Lung 21 at 24 h, respectively. At the 24 h time point, clearance from secondary and nontarget tissues was also observed. Altogether, our data suggest that 1E7-Fc represents a platform technology that can be employed to potentially both image and treat MET-altered NSCLC.
PMID:38470611 | DOI:10.1021/acs.bioconjchem.4c00019
View details for PubMedID 38470611
-
More
-
A Phase 2 Randomized Clinical Trial Evaluating 4-Dimensional Computed Tomography Ventilation-Based Functional Lung Avoidance Radiation Therapy for Non-Small Cell Lung Cancer International journal of radiation oncology, biology, physics
Baschnagel AM, Flakus MJ, Wallat EM, Wuschner AE, Chappell RJ, Bayliss RA, Kimple RJ, Christensen GE, Reinhardt JM, Bassetti MF, Bayouth JE
2024 Aug 1;119(5):1393-1402. doi: 10.1016/j.ijrobp.2024.02.019. Epub 2024 Feb 20.
-
More
PURPOSE: To determine whether 4-dimensional computed tomography (4DCT) ventilation-based functional lung avoidance radiation therapy preserves pulmonary function compared with standard radiation therapy for non-small cell lung cancer (NSCLC).
METHODS AND MATERIALS: This single center, randomized, phase 2 trial enrolled patients with NSCLC receiving curative intent radiation therapy with either stereotactic body radiation therapy or conventionally fractionated radiation therapy between 2016 and 2022. Patients were randomized 1:1 to standard of care radiation therapy or functional lung avoidance radiation therapy. The primary endpoint was the change in Jacobian-based ventilation as measured on 4DCT from baseline to 3 months postradiation. Secondary endpoints included changes in volume of high- and low-ventilating lung, pulmonary toxicity, and changes in pulmonary function tests (PFTs).
RESULTS: A total of 122 patients were randomized and 116 were available for analysis. Median follow up was 29.9 months. Functional avoidance plans significantly (P < .05) reduced dose to high-functioning lung without compromising target coverage or organs at risk constraints. When analyzing all patients, there was no difference in the amount of lung showing a reduction in ventilation from baseline to 3 months between the 2 arms (1.91% vs 1.87%; P = .90). Overall grade ≥2 and grade ≥3 pulmonary toxicities for all patients were 24.1% and 8.6%, respectively. There was no significant difference in pulmonary toxicity or changes in PFTs between the 2 study arms. In the conventionally fractionated cohort, there was a lower rate of grade ≥2 pneumonitis (8.2% vs 32.3%; P = .049) and less of a decline in change in forced expiratory volume in 1 second (-3 vs -5; P = .042) and forced vital capacity (1.5 vs -6; P = .005) at 3 months, favoring the functional avoidance arm.
CONCLUSIONS: There was no difference in posttreatment ventilation as measured by 4DCT between the arms. In the cohort of patients treated with conventionally fractionated radiation therapy with functional lung avoidance, there was reduced pulmonary toxicity, and less decline in PFTs suggesting a clinical benefit in patients with locally advanced NSCLC.
PMID:38387810 | DOI:10.1016/j.ijrobp.2024.02.019
View details for PubMedID 38387810
-
More
-
Network analyses: Inhibition of androgen receptor signaling reduces inflammation in the lung through AR-MAF-IL6 signaling axes Genes & diseases
Wang AR, Baschnagel AM, Ni Z, Brennan SR, Newton HK, Buehler D, Kendziorski C, Kimple RJ, Iyer G
2023 Aug 18;11(3):101072. doi: 10.1016/j.gendis.2023.07.001. eCollection 2024 May.
-
More
PMID:38292196 | PMC:PMC10825295 | DOI:10.1016/j.gendis.2023.07.001
View details for PubMedID 38292196
-
More
-
GABA(A) receptor activation drives GABARAP-Nix mediated autophagy to radiation-sensitize primary and brain-metastatic lung adenocarcinoma tumors bioRxiv : the preprint server for biology
Bhattacharya D, Barille R, Toukam DK, Gawali VS, Kallay L, Ahmed T, Brown H, Rezvanian S, Karve A, Desai PB, Medvedovic M, Wang K, Ionascu D, Harun N, Wang C, Baschnagel AM, Kritzer JA, Cook JM, Krummel AP, Sengupta S
2023 Dec 1:2023.11.29.569295. doi: 10.1101/2023.11.29.569295.
-
More
In non-small cell lung cancer (NSCLC) treatment, targeted therapies benefit only a subset of NSCLC, while radiotherapy responses are not durable and toxicity limits therapy. We find that a GABA(A) receptor activator, AM-101, impairs viability and clonogenicity of NSCLC primary and brain metastatic cells. Employing an ex vivo 'chip', AM-101 is as efficacious as the chemotherapeutic docetaxel, which is used with radiotherapy for advanced-stage NSCLC. In vivo , AM-101 potentiates radiation, including conferring a survival benefit to mice bearing NSCLC intracranial tumors. GABA(A) receptor activation stimulates a selective-autophagic response via multimerization of GABA(A) Receptor-Associated Protein (GABARAP), stabilization of mitochondrial receptor Nix, and utilization of ubiquitin-binding protein p62. A targeted-peptide disrupting Nix binding to GABARAP inhibits AM-101 cytotoxicity. This supports a model of GABA(A) receptor activation driving a GABARAP-Nix multimerization axis triggering autophagy. In patients receiving radiotherapy, GABA(A) receptor activation may improve tumor control while allowing radiation dose de-intensification to reduce toxicity.
HIGHLIGHTS: Activating GABA(A) receptors intrinsic to lung primary and metastatic brain cancer cells triggers a cytotoxic response. GABA(A) receptor activation works as well as chemotherapeutic docetaxel in impairing lung cancer viability ex vivo . GABA(A) receptor activation increases survival of mice bearing lung metastatic brain tumors.A selective-autophagic response is stimulated by GABA(A) receptor activation that includes multimerization of GABARAP and Nix.Employing a new nanomolar affinity peptide that abrogates autophagosome formation inhibits cytotoxicity elicited by GABA(A) receptor activation.
PMID:38076805 | PMC:PMC10705483 | DOI:10.1101/2023.11.29.569295
View details for PubMedID 38076805
-
More
-
Combining Dual Checkpoint Immunotherapy with Ablative Radiation to All Sites of Oligometastatic Non-Small Cell Lung Cancer: Toxicity and Efficacy Results of a Phase 1b Trial International journal of radiation oncology, biology, physics
Bassetti MF, Morris BA, Sethakorn N, Lang JM, Schehr JL, Zhao SG, Morris ZS, Buehler D, Eickhoff JC, Harari PM, Traynor AM, Campbell TC, Baschnagel AM, Leal TA
2024 Apr 1;118(5):1481-1489. doi: 10.1016/j.ijrobp.2023.11.040. Epub 2023 Dec 8.
-
More
PURPOSE: Ablative local treatment of all radiographically detected metastatic sites in patients with oligometastatic non-small cell lung cancer (NSCLC) increases progression-free survival (PFS) and overall survival (OS). Prior studies demonstrated the safety of combining stereotactic body radiation therapy (SBRT) with single-agent immunotherapy. We investigated the safety of combining SBRT to all metastatic tumor sites with dual checkpoint, anticytotoxic T-lymphocyte-associated protein 4 (anti-CTLA-4), and anti-programmed cell death ligand 1 (anti-PD-L1) immunotherapy for patients with oligometastatic NSCLC.
METHODS AND MATERIALS: We conducted a phase 1b clinical trial in patients with oligometastatic NSCLC with up to 6 sites of extracranial metastatic disease. All sites of disease were treated with SBRT to a dose of 30 to 50 Gy in 5 fractions. Dual checkpoint immunotherapy was started 7 days after completion of radiation using anti-CTLA-4 (tremelimumab) and anti-PD-L1 (durvalumab) immunotherapy for a total of 4 cycles followed by durvalumab alone until progression or toxicity.
RESULTS: Of the 17 patients enrolled in this study, 15 patients received at least 1 dose of combination immunotherapy per protocol. The study was closed early (17 of planned 21 patients) due to slow accrual during the COVID-19 pandemic. Grade 3+ treatment-related adverse events were observed in 6 patients (40%), of which only one was possibly related to the addition of SBRT to immunotherapy. Median PFS was 42 months and median OS has not yet been reached.
CONCLUSIONS: Delivering ablative SBRT to all sites of metastatic disease in combination with dual checkpoint immunotherapy did not result in excessive rates of toxicity compared with historical studies of dual checkpoint immunotherapy alone. Although the study was not powered for treatment efficacy results, durable PFS and OS results suggest potential therapeutic benefit compared with immunotherapy or radiation alone in this patient population.
PMID:38072321 | PMC:PMC10947887 | DOI:10.1016/j.ijrobp.2023.11.040
View details for PubMedID 38072321
-
More
-
MET Inhibitor Capmatinib Radiosensitizes MET Exon 14-Mutated and MET-Amplified Non-Small Cell Lung Cancer International journal of radiation oncology, biology, physics
Ramesh S, Cifci A, Javeri S, Minne RL, Longhurst CA, Nickel KP, Kimple RJ, Baschnagel AM
2024 Apr 1;118(5):1379-1390. doi: 10.1016/j.ijrobp.2023.11.013. Epub 2023 Nov 16.
-
More
PURPOSE: The objective of this study was to investigate the effects of inhibiting the MET receptor with capmatinib, a potent and clinically relevant ATP-competitive tyrosine kinase inhibitor, in combination with radiation in MET exon 14-mutated and MET-amplified non-small cell lung (NSCLC) cancer models.
METHODS AND MATERIALS: In vitro effects of capmatinib and radiation on cell proliferation, colony formation, MET signaling, apoptosis, and DNA damage repair were evaluated. In vivo tumor responses were assessed in cell line xenograft and patient-derived xenograft models. Immunohistochemistry was used to confirm the in vitro results.
RESULTS: In vitro clonogenic survival assays demonstrated radiosensitization with capmatinib in both MET exon 14-mutated and MET-amplified NSCLC cell lines. No radiation-enhancing effect was observed in MET wild-type NSCLC and a human bronchial epithelial cell line. Minimal apoptosis was detected with the combination of capmatinib and radiation. Capmatinib plus radiation compared with radiation alone resulted in inhibition of DNA double-strand break repair, as measured by prolonged expression of γH2AX. In vivo, the combination of capmatinib and radiation significantly delayed tumor growth compared with vehicle control, capmatinib alone, or radiation alone. Immunohistochemistry indicated inhibition of phospho-MET and phospho-S6 and a decrease in Ki67 with inhibition of MET.
CONCLUSIONS: Inhibition of MET with capmatinib enhances the effect of radiation in both MET exon 14-mutated and MET-amplified NSCLC models.
PMID:37979706 | DOI:10.1016/j.ijrobp.2023.11.013
View details for PubMedID 37979706
-
More
-
MET Inhibitor Capmatinib Radiosensitizes MET Exon 14-Mutated and MET-Amplified Non-Small Cell Lung Cancer bioRxiv : the preprint server for biology
Ramesh S, Cifci A, Javeri S, Minne R, Longhurst CA, Nickel KP, Kimple RJ, Baschnagel AM
2023 Oct 27:2023.10.26.564232. doi: 10.1101/2023.10.26.564232.
-
More
PURPOSE: The objective of this study was to investigate the effects of inhibiting the MET receptor with capmatinib, a potent and clinically relevant ATP-competitive tyrosine kinase inhibitor, in combination with radiation in MET exon 14-mutated and MET-amplified non-small cell lung (NSCLC) cancer models.
METHODS AND MATERIALS: In vitro effects of capmatinib and radiation on cell proliferation, colony formation, MET signaling, apoptosis, and DNA damage repair were evaluated. In vivo tumor responses were assessed in cell line xenograft and patient-derived xenograft models. Immunohistochemistry (IHC) was used to confirm in vitro results.
RESULTS: In vitro clonogenic survival assays demonstrated radiosensitization with capmatinib in both MET exon 14-mutated and MET-amplified NSCLC cell lines. No radiation-enhancing effect was observed in MET wild-type NSCLC and human bronchial epithelial cell line. Minimal apoptosis was detected with the combination of capmatinib and radiation. Capmatinib plus radiation compared to radiation alone resulted in inhibition of DNA double-strand break repair as measured by prolonged expression of γH2AX. In vivo, the combination of capmatinib and radiation significantly delayed tumor growth compared to vehicle control, capmatinib alone, or radiation alone. IHC indicated inhibition of phospho-MET and phospho-S6 and a decrease in Ki67 with inhibition of MET.
CONCLUSIONS: Inhibition of MET with capmatinib enhanced the effect of radiation in both MET exon 14-mutated and MET-amplified NSCLC models.
PMID:37961176 | PMC:PMC10634863 | DOI:10.1101/2023.10.26.564232
View details for PubMedID 37961176
-
More
-
Toxicity and Patient-Reported Quality-of-Life Outcomes After Prostate Stereotactic Body Radiation Therapy With Focal Boost to Magnetic Resonance Imaging-Identified Prostate Cancer Lesions: Results of a Phase 2 Trial International journal of radiation oncology, biology, physics
Morris BA, Holmes EE, Anger NJ, Cooley G, Schuster JM, Hurst N, Baschnagel AM, Bassetti MF, Blitzer GC, Chappell RJ, Bayliss RA, Morris ZS, Ritter MA, Floberg JM
2023 Nov 1;117(3):613-623. doi: 10.1016/j.ijrobp.2023.05.004. Epub 2023 May 12.
-
More
PURPOSE: In this prospective phase 2 trial, we investigated the toxicity and patient-reported quality-of-life outcomes in patients treated with stereotactic body radiation therapy (SBRT) to the prostate gland and a simultaneous focal boost to magnetic resonance imaging (MRI)-identified intraprostatic lesions while also de-escalating dose to the adjacent organs at risk.
METHODS AND MATERIALS: Eligible patients included low- or intermediate-risk prostate cancer (Gleason score ≤7, prostate specific antigen ≤20, T stage ≤2b). SBRT was prescribed to 40 Gy in 5 fractions delivered every other day to the prostate, with any areas of high disease burden (MRI-identified prostate imaging reporting and data system 4 or 5 lesions) simultaneously escalated to 42.5 to 45 Gy and areas overlapping organs at risk (within 2 mm of urethra, rectum, and bladder) constrained to 36.25 Gy (n = 100). Patients without a pretreatment MRI or without MRI-identified lesions were treated to dose of 37.5 Gy with no focal boost (n = 14).
RESULTS: From 2015 to 2022, a total of 114 patients were enrolled with a median follow-up of 42 months. No acute or late grade 3+ gastrointestinal (GI) toxicity was observed. One patient developed late grade 3 genitourinary (GU) toxicity at 16 months. In patients treated with focal boost (n = 100), acute grade 2 GU and GI toxicity was seen in 38% and 4% of patients, respectively. Cumulative late grade 2+ GU and GI toxicities at 24 months were 13% and 5% respectively. Patient-reported outcomes showed no significant long-term change from baseline in urinary, bowel, hormonal, or sexual quality-of-life scores after treatment.
CONCLUSIONS: SBRT to a dose of 40 Gy to the prostate gland with a simultaneous focal boost up to 45 Gy is well tolerated with similar rates of acute and late grade 2+ GI and GU toxicity as seen in other SBRT regimens without intraprostatic boost. Moreover, no significant long-term changes were seen in patient-reported urinary, bowel, or sexual outcomes from pretreatment baseline.
PMID:37179035 | DOI:10.1016/j.ijrobp.2023.05.004
View details for PubMedID 37179035
-
More
-
Sustained Preservation of Cognition and Prevention of Patient-Reported Symptoms With Hippocampal Avoidance During Whole-Brain Radiation Therapy for Brain Metastases: Final Results of NRG Oncology CC001 International journal of radiation oncology, biology, physics
Gondi V, Deshmukh S, Brown PD, Wefel JS, Armstrong TS, Tome WA, Gilbert MR, Konski A, Robinson CG, Bovi JA, Benzinger LS, Roberge D, Kundapur V, Kaufman I, Shah S, Usuki KY, Baschnagel AM, Mehta MP, Kachnic LA
2023 Nov 1;117(3):571-580. doi: 10.1016/j.ijrobp.2023.04.030. Epub 2023 May 6.
-
More
PURPOSE: Initial report of NRG Oncology CC001, a phase 3 trial of whole-brain radiation therapy plus memantine (WBRT + memantine) with or without hippocampal avoidance (HA), demonstrated neuroprotective effects of HA with a median follow-up of fewer than 8 months. Herein, we report the final results with complete cognition, patient-reported outcomes, and longer-term follow-up exceeding 1 year.
METHODS AND MATERIALS: Adult patients with brain metastases were randomized to HA-WBRT + memantine or WBRT + memantine. The primary endpoint was time to cognitive function failure, defined as decline using the reliable change index on the Hopkins Verbal Learning Test-Revised (HVLT-R), Controlled Oral Word Association, or the Trail Making Tests (TMT) A and B. Patient-reported symptom burden was assessed using the MD Anderson Symptom Inventory with Brain Tumor Module and EQ-5D-5L.
RESULTS: Between July 2015 and March 2018, 518 patients were randomized. The median follow-up for living patients was 12.1 months. The addition of HA to WBRT + memantine prevented cognitive failure (adjusted hazard ratio, 0.74, P = .016) and was associated with less deterioration in TMT-B at 4 months (P = .012) and HVLT-R recognition at 4 (P = .055) and 6 months (P = .011). Longitudinal modeling of imputed data showed better preservation of all HVLT-R domains (P < .005). Patients who received HA-WBRT + Memantine reported less symptom burden at 6 (P < .001 using imputed data) and 12 months (P = .026 using complete-case data; P < .001 using imputed data), less symptom interference at 6 (P = .003 using complete-case data; P = .0016 using imputed data) and 12 months (P = .0027 using complete-case data; P = .0014 using imputed data), and fewer cognitive symptoms over time (P = .043 using imputed data). Treatment arms did not differ significantly in overall survival, intracranial progression-free survival, or toxicity.
CONCLUSIONS: With median follow-up exceeding 1 year, HA during WBRT + memantine for brain metastases leads to sustained preservation of cognitive function and continued prevention of patient-reported neurologic symptoms, symptom interference, and cognitive symptoms with no difference in survival or toxicity.
PMID:37150264 | PMC:PMC11070071 | DOI:10.1016/j.ijrobp.2023.04.030
View details for PubMedID 37150264
-
More
-
Metrics of dose to highly ventilated lung are predictive of radiation-induced pneumonitis in lung cancer patients Radiotherapy and oncology : journal of the European Society for Therapeutic Radiology and Oncology
Flakus MJ, Kent SP, Wallat EM, Wuschner AE, Tennant E, Yadav P, Burr A, Yu M, Christensen GE, Reinhardt JM, Bayouth JE, Baschnagel AM
2023 May;182:109553. doi: 10.1016/j.radonc.2023.109553. Epub 2023 Feb 20.
-
More
PURPOSE: To identify metrics of radiation dose delivered to highly ventilated lung that are predictive of radiation-induced pneumonitis.
METHODS AND MATERIALS: A cohort of 90 patients with locally advanced non-small cell lung cancer treated with standard fractionated radiation therapy (RT) (60-66 Gy in 30-33 fractions) were evaluated. Regional lung ventilation was determined from pre-RT 4-dimensional computed tomography (4DCT) using the Jacobian determinant of a B-spline deformable image registration to estimate lung tissue expansion during respiration. Multiple voxel-wise population- and individual-based thresholds for defining high functioning lung were considered. Mean dose and volumes receiving dose ≥ 5-60 Gy were analyzed for both total lung-ITV (MLD,V5-V60) and highly ventilated functional lung-ITV (fMLD,fV5-fV60). The primary endpoint was symptomatic grade 2+ (G2+) pneumonitis. Receiver operator curve (ROC) analyses were used to identify predictors of pneumonitis.
RESULTS: G2+ pneumonitis occurred in 22.2% of patients, with no differences between stage, smoking status, COPD, or chemo/immunotherapy use between G<2 and G2+ patients (P≥ 0.18). Highly ventilated lung was defined as voxels exceeding the population-wide median of 18% voxel-level expansion. All total and functional metrics were significantly different between patients with and without pneumonitis (P≤ 0.039). Optimal ROC points predicting pneumonitis from functional lung dose were fMLD ≤ 12.3 Gy, fV5 ≤ 54% and fV20 ≤ 19 %. Patients with fMLD ≤ 12.3 Gy had a 14% risk of developing G2+ pneumonitis whereas risk significantly increased to 35% for those with fMLD > 12.3 Gy (P = 0.035).
CONCLUSIONS: Dose to highly ventilated lung is associated with symptomatic pneumonitis and treatment planning strategies should focus on limiting dose to functional regions. These findings provide important metrics to be used in functional lung avoidance RT planning and designing clinical trials.
PMID:36813178 | PMC:PMC10283046 | DOI:10.1016/j.radonc.2023.109553
View details for PubMedID 36813178
-
More
-
Dosimetric study for spine stereotactic body radiation therapy: magnetic resonance guided linear accelerator versus volumetric modulated arc therapy Radiology and oncology
Yadav P, Musunuru HB, Witt JS, Bassetti M, Bayouth J, Baschnagel AM
2022 Dec 13;56(4):553. doi: 10.2478/raon-2022-0044. eCollection 2022 Dec 1.
-
Radiomic Modeling of Bone Density and Rib Fracture Risk After Stereotactic Body Radiation Therapy for Early-Stage Non-Small Cell Lung Cancer Advances in radiation oncology
Rydzewski NR, Yadav P, Musunuru HB, Condit KM, Francis D, Zhao SG, Baschnagel AM
2021 Dec 29;7(3):100884. doi: 10.1016/j.adro.2021.100884. eCollection 2022 May-Jun.
-
More
PURPOSE: Our purpose was to determine whether bone density and bone-derived radiomic metrics in combination with dosimetric variables could improve risk stratification of rib fractures after stereotactic body radiation therapy (SBRT) for early-stage non-small cell lung cancer (NSCLC).
METHODS AND MATERIALS: A retrospective analysis was conducted of patients with early-stage NSCLC treated with SBRT. Dosimetric data and rib radiomic data extracted using PyRadiomics were used for the analysis. A subset of patients had bone density scans that were used to create a predicted bone density score for all patients. A 10-fold cross validated approach with 10 resamples was used to find the top univariate logistic models and elastic net regression models that predicted for rib fracture.
RESULTS: A total of 192 treatment plans were included in the study with a rib fracture rate of 16.1%. A predicted bone density score was created from a multivariate model with vertebral body Hounsfield units and patient weight, with an R-squared of 0.518 compared with patient dual-energy x-ray absorptiometry T-scores. When analyzing all patients, a low predicted bone density score approached significance for increased risk of rib fracture (P = .07). On competing risk analysis, when stratifying patients based on chest wall V30 Gy and bone density score, those with a V30 Gy ≥30 cc and a low bone density score had a significantly higher risk of rib fracture compared with all other patients (P < .001), with a predicted 2-year risk of rib fracture of 28.6% (95% confidence interval, 17.2%-41.1%) and 4.9% (95% confidence interval, 2.3%-9.0%), respectively. Dosimetric variables were the primary drivers of fracture risk. A multivariate elastic net regression model including all dosimetric variables was the best predictor of rib fracture (area under the curve [AUC], 0.864). Bone density variables (AUC, 0.618) and radiomic variables (AUC, 0.617) have better predictive power than clinical variables that exclude bone density (AUC, 0.538).
CONCLUSION: Radiomic features, including a bone density score that includes vertebral body Hounsfield units and radiomic signatures from the ribs, can be used to stratify risk of rib fracture after SBRT for NSCLC.
PMID:35647405 | PMC:PMC9133372 | DOI:10.1016/j.adro.2021.100884
View details for PubMedID 35647405
-
More
-
ATR Inhibitor M6620 (VX-970) Enhances the Effect of Radiation in Non-Small Cell Lung Cancer Brain Metastasis Patient-Derived Xenografts Molecular cancer therapeutics
Baschnagel AM, Elnaggar JH, VanBeek HJ, Kromke AC, Skiba JH, Kaushik S, Abel L, Clark PA, Longhurst CA, Nickel KP, Leal TA, Zhao SG, Kimple RJ
2021 Nov;20(11):2129-2139. doi: 10.1158/1535-7163.MCT-21-0305. Epub 2021 Aug 19.
-
More
M6620, a selective ATP-competitive inhibitor of the ATM and RAD3-related (ATR) kinase, is currently under investigation with radiation in patients with non-small cell lung cancer (NSCLC) brain metastases. We evaluated the DNA damage response (DDR) pathway profile of NSCLC and assessed the radiosensitizing effects of M6620 in a preclinical NSCLC brain metastasis model. Mutation analysis and transcriptome profiling of DDR genes and pathways was performed on NSCLC patient samples. NSCLC cell lines were assessed with proliferation, clonogenic survival, apoptosis, cell cycle, and DNA damage signaling and repair assays. NSCLC brain metastasis patient-derived xenograft models were used to assess intracranial response and overall survival. In vivo IHC was performed to confirm in vitro results. A significant portion of NSCLC patient tumors demonstrated enrichment of DDR pathways. DDR pathways correlated with lung squamous cell histology; and mutations in ATR, ATM, BRCA1, BRCA2, CHEK1, and CHEK2 correlated with enrichment of DDR pathways in lung adenocarcinomas. M6620 reduced colony formation after radiotherapy and resulted in inhibition of DNA DSB repair, abrogation of the radiation-induced G2 cell checkpoint, and formation of dysfunctional micronuclei, leading to enhanced radiation-induced mitotic death. The combination of M6620 and radiation resulted in improved overall survival in mice compared with radiation alone. In vivo IHC revealed inhibition of pChk1 in the radiation plus M6620 group. M6620 enhances the effect of radiation in our preclinical NSCLC brain metastasis models, supporting the ongoing clinical trial (NCT02589522) evaluating M6620 in combination with whole brain irradiation in patients with NSCLC brain metastases.
PMID:34413128 | PMC:PMC8571002 | DOI:10.1158/1535-7163.MCT-21-0305
View details for PubMedID 34413128
-
More
-
Radiation-induced Hounsfield unit change correlates with dynamic CT perfusion better than 4DCT-based ventilation measures in a novel-swine model Scientific reports
Wuschner AE, Wallat EM, Flakus MJ, Shanmuganayagam D, Meudt J, Christensen GE, Reinhardt JM, Miller JR, Lawless MJ, Baschnagel AM, Bayouth JE
2021 Jun 23;11(1):13156. doi: 10.1038/s41598-021-92609-x.
-
More
To analyze radiation induced changes in Hounsfield units and determine their correlation with changes in perfusion and ventilation. Additionally, to compare the post-RT changes in human subjects to those measured in a swine model used to quantify perfusion changes and validate their use as a preclinical model. A cohort of 5 Wisconsin Miniature Swine (WMS) were studied. Additionally, 19 human subjects were recruited as part of an IRB approved clinical trial studying functional avoidance radiation therapy for lung cancer and were treated with SBRT. Imaging (a contrast enhanced dynamic perfusion CT in the swine and 4DCT in the humans) was performed prior to and post-RT. Jacobian elasticity maps were calculated on all 4DCT images. Contours were created from the isodose lines to discretize analysis into 10 Gy dose bins. B-spline deformable image registration allowed for voxel-by-voxel comparative analysis in these contours between timepoints. The WMS underwent a research course of 60 Gy in 5 fractions delivered locally to a target in the lung using an MRI-LINAC system. In the WMS subjects, the dose-bin contours were copied onto the contralateral lung, which received < 5 Gy for comparison. Changes in HU and changes in Jacobian were analyzed in these contours. Statistically significant (p < 0.05) changes in the mean HU value post-RT compared to pre-RT were observed in both the human and WMS groups at all timepoints analyzed. The HU increased linearly with dose for both groups. Strong linear correlation was observed between the changes seen in the swine and humans (Pearson coefficient > 0.97, p < 0.05) at all timepoints. Changes seen in the swine closely modeled the changes seen in the humans at 12 months post RT (slope = 0.95). Jacobian analysis showed between 30 and 60% of voxels were damaged post-RT. Perfusion analysis in the swine showed a statistically significant (p < 0.05) reduction in contrast inside the vasculature 3 months post-RT compared to pre-RT. The increases in contrast outside the vasculature was strongly correlated (Pearson Correlation 0.88) with the reduction in HU inside the vasculature but were not correlated with the changes in Jacobians. Radiation induces changes in pulmonary anatomy at 3 months post-RT, with a strong linear correlation with dose. The change in HU seen in the non-vessel lung parenchyma suggests this metric is a potential biomarker for change in perfusion. Finally, this work suggests that the WMS swine model is a promising pre-clinical model for analyzing radiation-induced changes in humans and poses several benefits over conventional swine models.
PMID:34162987 | PMC:PMC8222280 | DOI:10.1038/s41598-021-92609-x
View details for PubMedID 34162987
-
More
-
Impact of immediate cryopreservation on the establishment of patient derived xenografts from head and neck cancer patients Journal of translational medicine
Abel L, Durmaz A, Hu R, Longhurst C, Baschnagel AM, Wheeler D, Scott JG, Kimple RJ
2021 Apr 28;19(1):180. doi: 10.1186/s12967-021-02850-1.
-
More
BACKGROUND: Patient-derived xenografts established from human cancers are important tools for investigating novel anti-cancer therapies. Establishing PDXs requires a significant investment and many PDXs may be used infrequently due to their similarity to existing models, their growth rate, or the lack of relevant mutations. We performed this study to determine whether we could efficiently establish PDXs after cryopreservation to allow molecular profiling to be completed prior to implanting the human cancer.
METHODS: Fresh tumor was split with half used to establish a PDX immediately and half cryopreserved for later implantation. Resulting tumors were assessed histologically and tumors established from fresh or cryopreserved tissues compared as to the growth rate, extent of tumor necrosis, mitotic activity, keratinization, and grade. All PDXs were subjected to short tandem repeat testing to confirm identity and assess similarity between methods.
RESULTS: Tumor growth was seen in 70% of implanted cases. No growth in either condition was seen in 30% of tumors. One developed a SCC from the immediate implant but a lymphoproliferative mass without SCC from the cryopreserved specimen. No difference in growth rate was seen. No difference between histologic parameters was seen between the two approaches.
CONCLUSIONS: Fresh human cancer tissue can be immediately cryopreserved and later thawed and implanted to establish PDXs. This resource saving approach allows for tumor profiling prior to implantation into animals thus maximizing the probability that the tumor will be utilized for future research.
PMID:33910584 | PMC:PMC8082827 | DOI:10.1186/s12967-021-02850-1
View details for PubMedID 33910584
-
More
-
Development and characterization of patient-derived xenografts from non-small cell lung cancer brain metastases Scientific reports
Baschnagel AM, Kaushik S, Durmaz A, Goldstein S, Ong IM, Abel L, Clark PA, Gurel Z, Leal T, Buehler D, Iyer G, Scott JG, Kimple RJ
2021 Jan 28;11(1):2520. doi: 10.1038/s41598-021-81832-1.
-
More
Non-small cell lung cancer (NSCLC) brain metastasis cell lines and in vivo models are not widely accessible. Herein we report on a direct-from patient-derived xenograft (PDX) model system of NSCLC brain metastases with genomic annotation useful for translational and mechanistic studies. Both heterotopic and orthotopic intracranial xenografts were established and RNA and DNA sequencing was performed on patient and matching tumors. Morphologically, strong retention of cytoarchitectural features was observed between original patient tumors and PDXs. Transcriptome and mutation analysis revealed high correlation between matched patient and PDX samples with more than more than 95% of variants detected being retained in the matched PDXs. PDXs demonstrated response to radiation, response to selumetinib in tumors harboring KRAS G12C mutations and response to savolitinib in a tumor with MET exon 14 skipping mutation. Savolitinib also demonstrated in vivo radiation enhancement in our MET exon 14 mutated PDX. Early passage cell strains showed high consistency between patient and PDX tumors. Together, these data describe a robust human xenograft model system for investigating NSCLC brain metastases. These PDXs and cell lines show strong phenotypic and molecular correlation with the original patient tumors and provide a valuable resource for testing preclinical therapeutics.
PMID:33510214 | PMC:PMC7843608 | DOI:10.1038/s41598-021-81832-1
View details for PubMedID 33510214
-
More
-
Combined Immunotherapy and Stereotactic Radiotherapy Improves Neurologic Outcomes in Patients with Non-small-cell Lung Cancer Brain Metastases Clinical lung cancer
Enright TL, Witt JS, Burr AR, Yadav P, Leal T, Baschnagel AM
2021 Mar;22(2):110-119. doi: 10.1016/j.cllc.2020.10.014. Epub 2020 Nov 10.
-
More
BACKGROUND: The purpose of this study was to compare the outcomes of patients with non-small cell lung cancer (NSCLC) brain metastases treated with stereotactic radiotherapy (SRT) alone versus SRT and immune checkpoint inhibitors (ICIs).
PATIENTS AND METHODS: Patients treated for their first diagnosis of intracranial metastases with SRT or SRT plus ICI were retrospectively identified. Overall survival (OS), local control (LC), distant brain failure (DBF), neurologic death, and rates of radiation necrosis were calculated. Univariate (UVA) and multivariable (MVA) analyses with competing risk analysis were performed.
RESULTS: Seventy-seven patients with 132 lesions were analyzed, including 44 patients with 68 lesions in the SRT group and 33 patients with 64 lesions in the SRT plus ICI group. There were no differences in baseline factors between groups. Use of ICI predicted for decreased DBF (hazard ratio [HR], 0.45; 95% confidence interval [CI], 0.24-0.84; P = .01), decreased rates of neurologic death (HR, 0.29; 95% CI, 0.10-0.85; P = .02), and better OS (HR, 0.46; 95% CI, 0.23-0.91; P = .03). Two-year LC was 97% for the SRT + ICI group, and 86% for the SRT-alone group (P = .046). Actuarial 2-year DBF was 39% for the SRT + ICI group and 66% for the SRT alone group (P = .016). On MVA, ICI use persisted in predicting lower incidence of neurologic death (HR, 0.25; 95% CI, 0.09-0.72; P = .01) and DBF (HR, 0.47; 95% CI, 0.25-0.85; P = .01) when adjusted for competing risk of death.
CONCLUSION: In this cohort of patients with NSCLC brain metastases, ICI use combined with SRT predicted for improved LC and OS and decreased DBF and risk of neurologic death.
PMID:33281062 | DOI:10.1016/j.cllc.2020.10.014
View details for PubMedID 33281062
-
More
-
Outcomes From Whole-Brain Reirradiation Using Pulsed Reduced Dose Rate Radiation Therapy Advances in radiation oncology
Burr AR, Robins HI, Bayliss RA, Baschnagel AM, Welsh JS, Tomé WA, Howard SP
2020 Jul 8;5(5):834-839. doi: 10.1016/j.adro.2020.06.021. eCollection 2020 Sep-Oct.
-
More
PURPOSE: Recurrent intracranial metastases after whole-brain irradiation pose a clinical challenge owing to the escalating morbidity associated with their treatment. Although stereotactic radiosurgery is increasingly being used, there are still situations in which whole-brain reirradiation (ReRT) continues to be appropriate. Here, we report our experience using whole-brain pulsed reduced dose rate radiation therapy (PRDR), a method that delivers radiation at a slower rate of 0.067 Gy/min to potentially increase sublethal damage repair and decrease toxicity.
METHODS AND MATERIALS: Patients undergoing whole-brain ReRT with PRDR from January 1, 2001 to March 2019 were analyzed. The median PRDR ReRT dose was 26 Gy in 2 Gy fractions, resulting in a median total whole-brain dose of 59.5 Gy. Cox regression analysis was used for multivariate analysis. The Kaplan-Meier method was used for overall survival, progression free survival, and to evaluate the ReRT score. Binary logistic regression was employed to evaluate variables associated with rapid death.
RESULTS: Seventy-five patients were treated with whole-brain PRDR radiation therapy. The median age was 54 (range, 26-72), the median Karnofsky performance status (KPS) was 80, and 86.7% had recursive partitioning analysis scores of 2. Thirty-two patients had over 10 metastases and 11 had leptomeningeal disease. The median overall survival was 4.1 months (range, 0.29-59.5 months) with a 1 year overall survival of 10.4%. Age, KPS, dexamethasone usage, and intracranial disease volume were significantly correlated with overall survival on multivariate analysis. A KPS ≤70 was associated with rapid death after radiation. The prognostic value of the ReRT score was validated. The most common acute toxicities were fatigue (23.1%) and headache (16.9%).
CONCLUSIONS: In this large cohort of patients with advanced intracranial metastases, PRDR achieves acceptable survival and may decrease toxicity associated with ReRT. PRDR is an easily implemented technique and is a viable treatment option for ReRT of brain metastases.
PMID:33083645 | PMC:PMC7557211 | DOI:10.1016/j.adro.2020.06.021
View details for PubMedID 33083645
-
More
-
Predictors of radiation necrosis in long-term survivors after Gamma Knife stereotactic radiosurgery for brain metastases Neuro-oncology practice
Siddiqui ZA, Squires BS, Johnson MD, Baschnagel AM, Chen PY, Krauss DJ, Olson RE, Meyer KD, Grills IS
2020 Jul;7(4):400-408. doi: 10.1093/nop/npz067. Epub 2019 Dec 6.
-
More
BACKGROUND: The long-term risk of necrosis after radiosurgery for brain metastases is uncertain. We aimed to investigate incidence and predictors of radiation necrosis for individuals with more than 1 year of survival after radiosurgery for brain metastases.
METHODS: Patients who had a diagnosis of brain metastases treated between December 2006 and December 2014, who had at least 1 year of survival after first radiosurgery were retrospectively reviewed. Survival was analyzed using the Kaplan-Meier estimator, and the incidence of radiation necrosis was estimated with death or surgical resection as competing risks. Patient and treatment factors associated with radiation necrosis were also analyzed.
RESULTS: A total of 198 patients with 732 lesions were analyzed. Thirty-four lesions required salvage radiosurgery and 10 required salvage surgical resection. Median follow-up was 24 months. The estimated median survival for this population was 25.4 months. The estimated per-lesion incidence of radiation necrosis at 4 years was 6.8%. Medical or surgical therapy was required for 60% of necrosis events. Tumor volume and male sex were significant factors associated with radiation necrosis. The per-lesions incidence of necrosis for patients undergoing repeat radiosurgery was 33.3% at 4 years.
CONCLUSIONS: In this large series of patients undergoing radiosurgery for brain metastases, patients continued to be at risk for radiation necrosis throughout their first 4 years of survival. Repeat radiosurgery of recurrent lesions greatly exacerbates the risk of radiation necrosis, whereas treatment of larger target volumes increases the risk modestly.
PMID:32765891 | PMC:PMC7393283 | DOI:10.1093/nop/npz067
View details for PubMedID 32765891
-
More
-
Prognostic factors and outcome of reirradiation for locally recurrent small cell lung cancer-a multicenter study Translational lung cancer research
Käsmann L, Janssen S, Baschnagel AM, Kruser TJ, Harada H, Aktan M, Rades D
2020 Apr;9(2):232-238. doi: 10.21037/tlcr.2020.01.19.
-
More
BACKGROUND: The prognosis of patients with recurrent small cell lung cancer (SCLC) remains poor and treatment options are limited. We performed a multi-institution retrospective cohort study to evaluate the outcome of thoracic reirradiation, identify prognostic factors and assess treatment-related toxicity.
METHODS: Data of 33 patients re-irradiated for recurrent SCLC at 4 international university hospitals, were analysed. Overall survival (OS) acute and late toxicities were evaluated and prognostic factors for reirradiation were identified.
RESULTS: Reirradiation (Re-RT) was performed at a median interval time of 24 months after the first thoracic radiotherapy series. Median survival after reirradiation was 7 months (range, 1-54 months). The Re-RT dose in EQD2 ranged from 20 to 87.50 Gy with a median of 32.50 Gy. The 1- and 2-year OS were 33% and 17%, respectively. Patients with a good performance status (KPS >70%), absence of extrathoracic disease, reirradiation dose (EQD2) of >40 Gy and a cumulative dose of first plus second series of radiotherapy (EQD2) >90 Gy were associated with improved OS. Acute pulmonary Grade 1-2 toxicity from re-irradiation was recorded in 11 patients (33%) and grade 3 acute toxicity was encountered 1 patient (3%).
CONCLUSIONS: Reirradiation for locoregionally recurrent SCLC is safe and shows promising outcomes. Patients reirradiated with doses >40 Gy experienced more favourable survival rates. In contrast, patients with a poor performance status or extrathoracic disease have a poor prognosis and Re-RT should be considered only for symptom control in this group.
PMID:32420062 | PMC:PMC7225148 | DOI:10.21037/tlcr.2020.01.19
View details for PubMedID 32420062
-
More
-
FGFR Inhibition Enhances Sensitivity to Radiation in Non-Small Cell Lung Cancer Molecular cancer therapeutics
SenthilKumar G, Fisher MM, Skiba JH, Miller MC, Brennan SR, Kaushik S, Bradley ST, Longhurst CA, Buehler D, Nickel KP, Iyer G, Kimple RJ, Baschnagel AM
2020 Jun;19(6):1255-1265. doi: 10.1158/1535-7163.MCT-19-0931. Epub 2020 May 5.
-
More
FGFRs are commonly altered in non-small cell lung cancer (NSCLC). FGFRs activate multiple pathways including RAS/RAF/MAPK, PI3K/AKT, and STAT, which may play a role in the cellular response to radiation. We investigated the effects of combining the selective FGFR 1-3 tyrosine kinase inhibitor AZD4547 with radiation in cell line and xenograft models of NSCLC. NSCLC cell lines were assessed with proliferation, clonogenic survival, apoptosis, autophagy, cell cycle, and DNA damage signaling and repair assays. In vivo xenografts and IHC were used to confirm in vitro results. NSCLC cell lines demonstrated varying degrees of FGFR protein and mRNA expression. In vitro clonogenic survival assays showed radiosensitization with AZD4547 in two NSCLC cell lines. In these two cell lines, an increase in apoptosis and autophagy was observed with combined radiation and AZD4547. The addition of AZD4547 to radiation did not significantly affect γH2AX foci formation. Enhanced xenograft tumor growth delay was observed with the combination of radiation and AZD4547 compared with radiation or drug alone. IHC results revealed inhibition of pMAPK and pS6 and demonstrated an increase in apoptosis in the radiation plus AZD4547 group. This study demonstrates that FGFR inhibition by AZD4547 enhances the response of radiation in FGFR-expressing NSCLC in vitro and in vivo model systems. These results support further investigation of combining FGFR inhibition with radiation as a clinical therapeutic strategy.
PMID:32371583 | PMC:PMC7272291 | DOI:10.1158/1535-7163.MCT-19-0931
View details for PubMedID 32371583
-
More
-
Fibroblast Growth Factor Receptors as Targets for Radiosensitization in Head and Neck Squamous Cell Carcinomas International journal of radiation oncology, biology, physics
Fisher MM, SenthilKumar G, Hu R, Goldstein S, Ong IM, Miller MC, Brennan SR, Kaushik S, Abel L, Nickel KP, Iyer G, Harari PM, Kimple RJ, Baschnagel AM
2020 Jul 15;107(4):793-803. doi: 10.1016/j.ijrobp.2020.03.040. Epub 2020 Apr 13.
-
More
PURPOSE: We examined the capacity of the pan-fibroblast growth factor receptor (FGFR) inhibitor AZD4547 to augment radiation response across a panel of head and neck squamous cell carcinoma (HNSCC) cell lines and xenografts.
METHODS AND MATERIALS: FGFR1, FGFR2, and FGFR3 RNA in situ hybridization expression was assessed in a cohort of HNSCC patient samples, cell lines, and patient-derived xenografts (PDXs). In vitro effects of AZD4547 and radiation on cell survival, FGFR signaling, apoptosis, autophagy, cell cycle, and DNA damage repair were evaluated. Reverse phase protein array was used to identify differentially phosphorylated proteins in cells treated with AZD4547. In vivo tumor responses were evaluated in cell lines and PDX models.
RESULTS: FGFR1, FGFR2, and FGFR3 RNA in situ hybridization were expressed in 41%, 81%, and 89% of 107 oropharynx patient samples. Sensitivity to AZD4547 did not directly correlate with FGFR protein or RNA expression. In sensitive cell lines, AZD4547 inhibited p-MAPK in a time-dependent manner. Significant radiosensitization with AZD4547 was observed in cell lines that were sensitive to AZD4547. The mechanism underlying these effects appears to be multifactorial, involving inhibition of the MTOR pathway and subsequent enhancement of autophagy and activation of apoptotic pathways. Significant tumor growth delay was observed when AZD4547 was combined with radiation compared with radiation or drug alone in an FGFR-expressing HNSCC cell line xenograft and PDX.
CONCLUSIONS: These findings suggest that AZD4547 can augment the response of radiation in FGFR-expressing HNSCC in vivo model systems. FGFR1 and FGFR2 may prove worthy targets for radiosensitization in HNSCC clinical investigations.
PMID:32298810 | PMC:PMC7321889 | DOI:10.1016/j.ijrobp.2020.03.040
View details for PubMedID 32298810
-
More
-
Modeling the impact of out-of-phase ventilation on normal lung tissue response to radiation dose Medical physics
Wallat EM, Flakus MJ, Wuschner AE, Shao W, Christensen GE, Reinhardt JM, Baschnagel AM, Bayouth JE
2020 Jul;47(7):3233-3242. doi: 10.1002/mp.14146. Epub 2020 Apr 13.
-
More
PURPOSE: To create a dose-response model that predicts lung ventilation change following radiation therapy, and examine the effects of out-of-phase ventilation.
METHODS: The dose-response model was built using 27 human subjects who underwent radiation therapy (RT) from an IRB-approved trial. For each four-dimensional computed tomography, two ventilation maps were created by calculating the N-phase local expansion ratio (LERN ) using most or all breathing phases and the 2-phase LER (LER2 ) using only the end inspiration and end expiration breathing phases. A polynomial regression model was created using the LERN ventilation maps pre-RT and post-RT and dose distributions for each subject, and crossvalidated with a leave-one-out method. Further validation of the model was performed using 15 additional human subjects using common statistical operating characteristics and gamma pass rates.
RESULTS: For voxels receiving 20 Gy or greater, there was a significant increase from 52% to 59% (P = 0.03) in the gamma pass rates of the LERN model predicted post-RT Jacobian maps to the actual post-RT Jacobian maps, relative to the LER2 model. Additionally, accuracy significantly increased (P = 0.03) from 68% to 75% using the LERN model, relative to the LER2 model.
CONCLUSIONS: The LERN model was significantly more accurate than the LER2 model at predicting post-RT ventilation maps. More accurate post-RT ventilation maps will aid in producing a higher quality functional avoidance treatment plan, allowing for potentially better normal tissue sparing.
PMID:32187683 | PMC:PMC9486957 | DOI:10.1002/mp.14146
View details for PubMedID 32187683
-
More
-
Prognostic significance of MTOR expression in HPV positive and negative head and neck cancers treated by chemoradiation Head & neck
Wilson TG, Hanna A, Recknagel J, Pruetz BL, Baschnagel AM, Wilson GD
2020 Feb;42(2):153-162. doi: 10.1002/hed.25983. Epub 2019 Oct 27.
-
More
BACKGROUND: The mechanistic target of rapamycin (MTOR) plays a key role in regulating cell growth and metabolism and is commonly overexpressed in head and neck cancer (HNSCC). This study investigated the association of MTOR with clinical outcome in human papilloma virus (HPV) positive and negative HNSCC patients treated by chemoradiation.
METHODS: A tissue microarray (TMA) consisting of cores from 109 HNSCC patients treated by definitive chemoradiation was constructed and stained with antibodies against p16 and MTOR and expression correlated with clinicopathological features and clinical outcome.
RESULTS: MTOR varied widely between tumor cores and was not associated with HPV status or clinicopathological features. There was a positive correlation with pre-treatment FDG uptake. (P = .01). In HPV negative patients, MTOR predicted for shorter locoregional control (P = .02), diseases free survival (P = .02), and overall survival (P = .04). MTOR expression was not associated with outcome in HPV positive patients.
CONCLUSIONS: Prognostic significance of MTOR expression depends on HPV status.
PMID:31657099 | DOI:10.1002/hed.25983
View details for PubMedID 31657099
-
More
-
Impact of adjuvant fractionated stereotactic radiotherapy dose on local control of brain metastases Journal of neuro-oncology
Musunuru HB, Witt JS, Yadav P, Francis DM, Kuczmarska-Haas A, Labby ZE, Bassetti MF, Howard SP, Baschnagel AM
2019 Nov;145(2):385-390. doi: 10.1007/s11060-019-03308-7. Epub 2019 Oct 12.
-
More
PURPOSE: The aim of this study was to determine whether a higher biological effective dose (BED) would result in improved local control in patients treated with fractionated stereotactic radiotherapy (FSRT) for their resected brain metastases.
METHODS: Patients with newly diagnosed brain metastases without previous brain radiotherapy were retrospectively reviewed. Patients underwent surgical resection of at least one brain metastasis and were treated with adjuvant FSRT, delivering 25-36 Gy in 5-6 fractions. Outcomes were computed using Kaplan-Meier survival analysis and univariate analysis.
RESULTS: Fifty-four patients with 63 post-operative cavities were included. Median follow-up was 16 months (3-60). Median metastasis size at diagnosis was 2.9 cm (0.6-8.1) and median planning target volume was 19.7 cm3 (6.3-68.1). Two-year local control (LC) was 83%. When stratified by dose, 2 years LC rate was 95.1% in those treated with 30-36 Gy in 5-6 fractions (BED10 of 48-57.6 Gy10) versus 59.1% lesions treated with 25 Gy in 5 fractions (BED10 of 37.5 Gy10) (p < 0.001). LC was not associated with resection cavity size. One year overall survival was 68.7%, and was independent of BED10. Symptomatic radiation necrosis occurred in 7.9% of patients and was not associated with dose.
CONCLUSION: In the post-operative setting, high-dose FSRT (BED10 > 37.5 Gy10) were associated with a significantly higher rate of LC compared to lower BED regimens. Overall, 25 Gy in 5 fractions is not an adequate dose to control microscopic disease. If selecting a 5-fraction regimen, 30 Gy in five fractions appears to provide excellent tumor bed control.
PMID:31606876 | DOI:10.1007/s11060-019-03308-7
View details for PubMedID 31606876
-
More
-
Dosimetric study for spine stereotactic body radiation therapy: magnetic resonance guided linear accelerator versus volumetric modulated arc therapy Radiology and oncology
Yadav P, Musunuru HB, Witt JS, Bassetti M, Bayouth J, Baschnagel AM
2019 Sep 24;53(3):362-368. doi: 10.2478/raon-2019-0042.
-
More
Background Stereotactic body radiation therapy (SBRT) given in 1-5 fractions is an effective treatment for vertebral metastases. Real-time magnetic resonance-guided radiotherapy (MRgRT) improves soft tissue contrast, which translates into accurate delivery of spine SBRT. Here we report on clinical implementation of MRgRT for spine SBRT, the quality of MRgRT plans compared to TrueBeam based volumetric modulated arc therapy (VMAT) plans in the treatment of spine metastases and benefits of MRgRT MR scan. Patients and methods Ten metastatic lesions were included in this study for plan comparison. Lesions were spread across thoracic spine and lumbosacral spine. Three fraction spine SBRT plans: 27Gy to planning target volume (PTV) and 30Gy to gross tumor volume (GTV) were generated on the ViewRay MRIdian Linac system and compared to TrueBeamTM STx based VMAT plans. Plans were compared using metrics such as minimum dose, maximum dose, mean dose, ratio of the dose to 50% of the volume (R50), conformity index, homogeneity index and dose to the spinal cord. Results MRIdian plans achieved equivalent target coverage and spinal cord dose compared to VMAT plans. The maximum and minimum PTV doses and homogeneity index were equivalent for both planning systems. R50 was lower for MRIdian plans compared to VMAT plans, indicating a lower spread of intermediate doses with MRIdian system (5.16 vs. 6.11, p = 0.03). Conclusions MRgRT can deliver high-quality spine SBRT plans comparable to TrueBeam volumetric modulated arc therapy (VMAT) plans.
PMID:31553704 | PMC:PMC6765155 | DOI:10.2478/raon-2019-0042
View details for PubMedID 31553704
-
More
-
Cardiac Toxicity in Operable Esophageal Cancer Patients Treated With or Without Chemoradiation American journal of clinical oncology
Witt JS, Jagodinsky JC, Liu Y, Yadav P, Kuczmarska-Haas A, Yu M, Maloney JD, Ritter MA, Bassetti MF, Baschnagel AM
2019 Aug;42(8):662-667. doi: 10.1097/COC.0000000000000573.
-
More
PURPOSE: The purpose of this study was to evaluate predictors of cardiac events in esophageal cancer patients treated with neoadjuvant chemoradiotherapy (NA CRT) followed by surgery compared with surgery alone.
MATERIALS AND METHODS: We retrospectively identified patients treated for esophageal cancer between 2006 and 2016. A total of 123 patients were identified; 70 were treated with surgery alone, and 53 were treated with NA CRT. Cardiac events were scored based on Common Terminology Criteria for Adverse Events (version 4.03), and dosimetric data was compiled for all patients who received radiation. Univariate analysis and multivariable analysis (MVA) were performed to identify predictors of cardiac events. Competing risk of death regression was performed to a model the cumulative incidence of cardiac events.
RESULTS: The overall rates of grade ≥3 cardiac events were 24.5% in the NA CRT group versus 10% in the surgery group (P=0.04). On MVA, use of NA CRT (P<0.01, hazard ratio [HR]: 3.45, 95% confidence interval [CI]: 1.35-9.09) predicted for grade ≥3 cardiac events, though no dosimetric variable predicted for grade ≥3 cardiac events or overall survival. On MVA, NA CRT predicted for pericardial effusions of any grade (P<0.01, HR: 3.70, 95% CI: 1.67-8.33). The V45 Gy was the most significant predictor of pericardial effusions (P=0.012, HR: 1.03, 95% CI: 1.01-1.06) CONCLUSIONS:: NA CRT significantly increased the rate of grade ≥3 cardiac events compared with patients treated with surgery alone. Although no dosimetric parameter predicted for grade ≥3 cardiac events or survival, the V45 Gy predicted for pericardial effusions.
PMID:31313677 | PMC:PMC6828548 | DOI:10.1097/COC.0000000000000573
View details for PubMedID 31313677
-
More
-
Pre-treatment serum bicarbonate predicts for primary tumor control after stereotactic body radiation therapy in patients with localized non-small cell lung cancer Radiotherapy and oncology : journal of the European Society for Therapeutic Radiology and Oncology
Sebastian N, Wu T, Driscoll E, Willers H, Kelly S, Musunuru HB, Mo X, Tan Y, Bazan J, Haglund K, Xu-Welliver M, Baschnagel AM, Ju A, Keane F, Williams TM
2019 Nov;140:26-33. doi: 10.1016/j.radonc.2019.05.014. Epub 2019 Jun 5.
-
More
BACKGROUND: Tumor aggressiveness and hypoxia are linked to acidosis in the tumor microenvironment (TME). We hypothesized that low pre-treatment serum bicarbonate, potentially correlating with an acidic and hypoxic TME, predicts for poor outcomes after stereotactic body radiation therapy (SBRT) for non-small cell lung cancer (NSCLC).
METHODS: We included patients with localized NSCLC treated to a biologically effective dose (BED) ≥ 100 Gy, with available pre-treatment bicarbonate values within 3 months of treatment. We used receiver operating characteristic analysis to determine the bicarbonate concentration optimally predicting for primary tumor recurrence, and evaluated its association with recurrence and survival. We validated our findings in an independent cohort of patients from three collaborating institutions.
RESULTS: A total of 110 patients and 114 tumors were included in the training cohort, with median follow-up of 15.0 months. Bicarbonate < 26 mEq/L was associated with primary tumor recurrence on univariate (HR = 5.92; 95% CI 1.69-24.88; p = 0.005) and multivariate analysis (HR = 5.48; 95% CI 1.37-25.19; p = 0.020). The validation cohort consisted of 195 patients and 208 tumors with median follow-up of 27.5 months. In the validation cohort, bicarbonate < 26 mEq/L was again associated with primary tumor recurrence on univariate (HR = 3.38; 95% CI 1.27-9.37; p = 0.015) and multivariate analysis (HR = 3.33; 1.18-10.07; p = 0.023).
CONCLUSIONS: Pre-treatment bicarbonate predicts for primary tumor control in NSCLC treated with SBRT and may be useful for risk stratification. These findings should be confirmed prospectively.
PMID:31176206 | PMC:PMC7080525 | DOI:10.1016/j.radonc.2019.05.014
View details for PubMedID 31176206
-
More
-
MR-based treatment planning in radiation therapy using a deep learning approach Journal of applied clinical medical physics
Liu F, Yadav P, Baschnagel AM, McMillan AB
2019 Mar;20(3):105-114. doi: 10.1002/acm2.12554.
-
More
PURPOSE: To develop and evaluate the feasibility of deep learning approaches for MR-based treatment planning (deepMTP) in brain tumor radiation therapy.
METHODS AND MATERIALS: A treatment planning pipeline was constructed using a deep learning approach to generate continuously valued pseudo CT images from MR images. A deep convolutional neural network was designed to identify tissue features in volumetric head MR images training with co-registered kVCT images. A set of 40 retrospective 3D T1-weighted head images was utilized to train the model, and evaluated in 10 clinical cases with brain metastases by comparing treatment plans using deep learning generated pseudo CT and using an acquired planning kVCT. Paired-sample Wilcoxon signed rank sum tests were used for statistical analysis to compare dosimetric parameters of plans made with pseudo CT images generated from deepMTP to those made with kVCT-based clinical treatment plan (CTTP).
RESULTS: deepMTP provides an accurate pseudo CT with Dice coefficients for air: 0.95 ± 0.01, soft tissue: 0.94 ± 0.02, and bone: 0.85 ± 0.02 and a mean absolute error of 75 ± 23 HU compared with acquired kVCTs. The absolute percentage differences of dosimetric parameters between deepMTP and CTTP was 0.24% ± 0.46% for planning target volume (PTV) volume, 1.39% ± 1.31% for maximum dose and 0.27% ± 0.79% for the PTV receiving 95% of the prescribed dose (V95). Furthermore, no significant difference was found for PTV volume (P = 0.50), the maximum dose (P = 0.83) and V95 (P = 0.19) between deepMTP and CTTP.
CONCLUSIONS: We have developed an automated approach (deepMTP) that allows generation of a continuously valued pseudo CT from a single high-resolution 3D MR image and evaluated it in partial brain tumor treatment planning. The deepMTP provided dose distribution with no significant difference relative to a kVCT-based standard volumetric modulated arc therapy plans.
PMID:30861275 | PMC:PMC6414148 | DOI:10.1002/acm2.12554
View details for PubMedID 30861275
-
More
-
The ASTRO Research Portfolio: Where Do We Go From Here? International journal of radiation oncology, biology, physics
Yu JB, Beck TF, Anscher MS, Baschnagel AM, Brock KK, Carlson DJ, Dominello MM, Kimple RJ, Knisely PS, Mendonca MS, Mian OY, Singh AK, Moros EG, Keen JC
2019 Feb 1;103(2):308-309. doi: 10.1016/j.ijrobp.2018.09.009.
-
Analysis of the 2017 American Society for Radiation Oncology (ASTRO) Research Portfolio International journal of radiation oncology, biology, physics
Yu JB, Beck TF, Anscher MS, Baschnagel AM, Brock KK, Carlson DJ, Dominello MM, Kimple RJ, Knisely JP, Mendonca MS, Mian OY, Singh AK, Moros EG, Keen JC
2019 Feb 1;103(2):297-304. doi: 10.1016/j.ijrobp.2018.07.2056.
-
More
PURPOSE: Research in radiation oncology (RO) is imperative to support the discovery of new uses of radiation and improvement of current approaches to radiation delivery and to foster the continued evolution of our field. Therefore, in 2016, the American Society of Radiation Oncology performed an evaluation of research grant funding for RO.
METHODS AND MATERIALS: Members of the Society of Chairs of Academic Radiation Oncology Programs (SCAROP) were asked about funded and unfunded grants that were submitted by their departments between the fiscal years 2014 and 2016. Grants were grouped according to broad categories defined by the 2017 American Society of Radiation Oncology Research Agenda. Additionally, active grants in the National Institutes of Health (NIH) Research Portfolio Online Reporting Tools database were collated using RO faculty names.
RESULTS: Overall, there were 816 funded (44%) and 1031 unfunded (56%) SCAROP-reported grants. Total grant funding was over $196 million. The US government funded the plurality (42.2%; 345 of 816) of grants compared with nonprofit and industry funders. Investigators from 10 institutions accounted for >75% of funded grants. Of the funded grants, 43.5% were categorized as "genomic influences and targeted therapies." The proportion of funded to unfunded grants was highest within the category of "tumor microenvironment, normal tissue effects, and reducing toxicity" (53.4% funded). "New clinical trial design and big data" had the smallest share of SCAROP grant applications and the lowest percent funded (38.3% of grants). NIH grants to RO researchers in 2014 to 2016 accounted for $85 million in funding. From the 31 responding SCAROP institutions, there was a 28% average success rate for RO proposals submitted to the NIH during this period.
CONCLUSIONS: Though RO researchers from responding institutions were relatively successful in obtaining funding, the overall amount awarded remains small. Continued advocacy on behalf of RO is needed, as well as investment to make research careers more attractive areas for emerging faculty.
PMID:30647006 | DOI:10.1016/j.ijrobp.2018.07.2056
View details for PubMedID 30647006
-
More
-
HPV impacts survival of stage IVC non-oropharyngeal HNSCC cancer patients Otorhinolaryngology-head and neck surgery
Burr AR, Harari PM, Ko HC, Chen S, Yu M, Baschnagel AM, Kimple RJ, Witek ME
2018;3(1):10.15761/OHNS.1000160. doi: 10.15761/OHNS.1000160. Epub 2018 Feb 24.
-
More
OBJECTIVES: Human papillomavirus (HPV) status is a favorable prognostic marker for patients with oropharyngeal squamous cell carcinoma (OPSCC) and non-metastatic head and neck non-OPSCC. We evaluated the impact of HPV status on overall survival (OS) for patients with Stage IVC non-OPSCC.
MATERIALS AND METHODS: Patients diagnosed with Stage IVC non-OPSCC and known HPV status between 2010-2013 were identified in the National Cancer Database. Univariate and multivariate analyses were performed to determine factors associated with OS. Propensity score-weighted Kaplan-Meier estimation was used to adjust for confounders in OS analyses. Multiple imputation method was used for sensitivity analysis.
RESULTS: We identified 708 patients with Stage IVC non-OPSCC with 30% being HPV-positive. Unadjusted median survival was 10.3 months for HPV-negative patients and 21.4 months for HPV-positive patients (p<0.0001). Age ≥ 65 and tumor diameter were associated with worse OS (p<0.05) while treatment versus no treatment and HPV-positive status were associated with improved OS on multivariate analysis (p<0.001). Adjusted median survival for patients with HPV-negative and HPV-positive disease was 11.1 months and 23.8 months, respectively (p<0.001). On unadjusted subgroup analysis, patients with HPV-positive oral cavity disease exhibited improved outcomes (p<0.0001) while HPV-positive hypopharynx (p<0.06) and larynx (p<0.12) patients exhibited a trend for improved OS compared to HPV-negative patients. The survival advantage associated with HPV positivity was maintained on sensitivity analysis (p<0.01).
CONCLUSION: These data demonstrate a clinically meaningful association between HPV status and OS in patients with non-OSPCC presenting with Stage IVC disease. In the absence of randomized data, these findings support active consideration of HPV status in clinical decision making, clinical trial design, and patient counseling regarding prognosis.
PMID:30271885 | PMC:PMC6157736 | DOI:10.15761/OHNS.1000160
View details for PubMedID 30271885
-
More
-
A New Era of Image Guidance with Magnetic Resonance-guided Radiation Therapy for Abdominal and Thoracic Malignancies Cureus
Mittauer K, Paliwal B, Hill P, Bayouth JE, Geurts MW, Baschnagel AM, Bradley KA, Harari PM, Rosenberg S, Brower JV, Wojcieszynski AP, Hullett C, Bayliss RA, Labby ZE, Bassetti MF
2018 Apr 4;10(4):e2422. doi: 10.7759/cureus.2422.
-
More
Magnetic resonance-guided radiation therapy (MRgRT) offers advantages for image guidance for radiotherapy treatments as compared to conventional computed tomography (CT)-based modalities. The superior soft tissue contrast of magnetic resonance (MR) enables an improved visualization of the gross tumor and adjacent normal tissues in the treatment of abdominal and thoracic malignancies. Online adaptive capabilities, coupled with advanced motion management of real-time tracking of the tumor, directly allow for high-precision inter-/intrafraction localization. The primary aim of this case series is to describe MR-based interventions for localizing targets not well-visualized with conventional image-guided technologies. The abdominal and thoracic sites of the lung, kidney, liver, and gastric targets are described to illustrate the technological advancement of MR-guidance in radiotherapy.
PMID:29872602 | PMC:PMC5985918 | DOI:10.7759/cureus.2422
View details for PubMedID 29872602
-
More
-
Impact of HPV Status on the Prognostic Potential of the AJCC Staging System for Larynx Cancer Otolaryngology--head and neck surgery : official journal of American Academy of Otolaryngology-Head and Neck Surgery
Davidson SM, Ko HC, Harari PM, Wieland AM, Chen S, Baschnagel AM, Kimple RJ, Witek ME
2018 Sep;159(3):456-465. doi: 10.1177/0194599818766035. Epub 2018 Apr 3.
-
More
Objective We evaluated the ability of the American Joint Committee on Cancer (AJCC) seventh edition staging system to prognosticate the overall survival of patients with human papillomavirus (HPV)-positive laryngeal squamous cell carcinoma. Study Design Retrospective analysis. Setting National Cancer Database. Subjects and Methods Patients diagnosed with laryngeal squamous cell carcinoma who were treated with curative intent were identified in the National Cancer Database. Multivariate analysis was utilized to determine factors correlated with overall survival in the HPV-negative and HPV-positive cohorts. Unadjusted and propensity score-weighted Kaplan-Meier estimation was used to determine overall survival of HPV-negative and HPV-positive patients across AJCC stage groupings. Results We identified 3238 patients with laryngeal squamous cell carcinoma, of which 2812 were HPV negative and 426 were HPV positive. Overall survival adjusted for age, sex, and comorbidity status confirmed significant differences among all consecutive stage groupings (I vs II, P < .001; II vs III, P < .05; III vs IVA, P < .001; IVA vs IVB, P < .05) in the HPV-negative cohort, whereas only stages IVAs and IVB ( P < .01) exhibited a significant difference in overall survival for HPV-positive patients. Conclusion The current AJCC staging system does not accurately distinguish risk of mortality for patients with HPV-positive disease. These data support the consideration of HPV status in estimating prognosis as well as clinical trial design and clinical decision making for patients with laryngeal squamous cell carcinoma.
PMID:29611770 | PMC:PMC7141595 | DOI:10.1177/0194599818766035
View details for PubMedID 29611770
-
More
-
Survival Outcomes for Patients With T3N0M0 Squamous Cell Carcinoma of the Glottic Larynx JAMA otolaryngology-- head & neck surgery
Ko HC, Harari PM, Chen S, Wieland AM, Yu M, Baschnagel AM, Kimple RJ, Witek ME
2017 Nov 1;143(11):1126-1133. doi: 10.1001/jamaoto.2017.1756.
-
More
IMPORTANCE: Radiotherapy (RT)-based organ preservation approaches for patients with advanced laryngeal cancer have been established stepwise through prospective randomized clinical trials. However, broad adoption of these approaches has stimulated discussion about long-term results challenging their applicability in a heterogeneous patient population, most recently for patients with T3 disease.
OBJECTIVE: To define outcomes in patients with clinical T3N0M0 glottic laryngeal cancer treated with definitive surgical and RT-based approaches.
DESIGN, SETTING, AND PARTICIPANTS: This retrospective cohort study included patients treated from January 1, 2004, through December 31, 2013, with a median follow-up time of 58 months (range, 0-126.6 months) in the National Cancer Database. Of the 4003 patients with T3N0M0 disease, 2622 received definitive therapy defined by the study protocol. Data were obtained from the clinical oncology database sourced from hospital registry data that are collected from more than 1500 Commission on Cancer-accredited facilities. Data were analyzed from September 14, 2016, through April 24, 2017.
INTERVENTIONS: Radiotherapy, chemoradiotherapy, surgery, surgery and RT, or surgery and chemoradiotherapy.
MAIN OUTCOMES AND MEASURES: Five-year overall survival (OS).
RESULTS: A total of 2622 patients (2251 men [85.9%] and 371 women [14.1%]; median age, 64 years [range, 19-90 years]) were included in the analytic cohort. In the overall patient cohort, the adjusted 5-year survival probability was 53%. No statistical differences were observed between the primary surgery (53%; 95% CI, 48%-57%) and primary RT (54%; 95% CI, 52%-57%) cohorts. In multivariate analysis, patient factors associated with decreased OS included age (hazard ratio [HR], 1.04; 95% CI, 1.03-1.04), insurance status (HR, 1.26; 95% CI, 1.06-1.50), and increasing comorbidity (HR, 1.20; 95% CI, 1.02-1.42).
CONCLUSIONS AND RELEVANCE: Current management of T3N0M0 glottic laryngeal cancer relies largely on RT-based organ preservation approaches. The present study substantiates randomized clinical trial data supporting the use of RT-based organ preservation approaches for patients with T3N0M0 glottic laryngeal cancer without compromising OS.
PMID:29049434 | PMC:PMC5710357 | DOI:10.1001/jamaoto.2017.1756
View details for PubMedID 29049434
-
More
-
Precision Oncology and Genomically Guided Radiation Therapy: A Report From the American Society for Radiation Oncology/American Association of Physicists in Medicine/National Cancer Institute Precision Medicine Conference International journal of radiation oncology, biology, physics
Hall WA, Bergom C, Thompson RF, Baschnagel AM, Vijayakumar S, Willers H, Li XA, Schultz CJ, Wilson GD, West ML, Capala J, Coleman CN, Torres-Roca JF, Weidhaas J, Feng FY
2018 Jun 1;101(2):274-284. doi: 10.1016/j.ijrobp.2017.05.044. Epub 2017 Jun 9.
-
More
PURPOSE: To summarize important talking points from a 2016 symposium focusing on real-world challenges to advancing precision medicine in radiation oncology, and to help radiation oncologists navigate the practical challenges of precision, radiation oncology.
METHODS AND MATERIALS: The American Society for Radiation Oncology, American Association of Physicists in Medicine, and National Cancer Institute cosponsored a meeting on precision medicine in radiation oncology. In June 2016 numerous scientists, clinicians, and physicists convened at the National Institutes of Health to discuss challenges and future directions toward personalized radiation therapy. Various breakout sessions were held to discuss particular components and approaches to the implementation of personalized radiation oncology. This article summarizes the genomically guided radiation therapy breakout session.
RESULTS: A summary of existing genomic data enabling personalized radiation therapy, ongoing clinical trials, current challenges, and future directions was collected. The group attempted to provide both a current overview of data that radiation oncologists could use to personalize therapy, along with data that are anticipated in the coming years. It seems apparent from the provided review that a considerable opportunity exists to truly bring genomically guided radiation therapy into clinical reality.
CONCLUSIONS: Genomically guided radiation therapy is a necessity that must be embraced in the coming years. Incorporating these data into treatment recommendations will provide radiation oncologists with a substantial opportunity to improve outcomes for numerous cancer patients. More research focused on this topic is needed to bring genomic signatures into routine standard of care.
PMID:28964588 | DOI:10.1016/j.ijrobp.2017.05.044
View details for PubMedID 28964588
-
More
-
Prognostic implications of human papillomavirus status for patients with non-oropharyngeal head and neck squamous cell carcinomas Journal of cancer research and clinical oncology
Ko HC, Harari PM, Sacotte RM, Chen S, Wieland AM, Yu M, Baschnagel AM, Bruce JY, Kimple RJ, Witek ME
2017 Nov;143(11):2341-2350. doi: 10.1007/s00432-017-2481-8. Epub 2017 Jul 27.
-
More
PURPOSE: We examined overall survival in a large cohort of patients with human papillomavirus (HPV)-positive and HPV-negative non-oropharyngeal squamous cell carcinoma of the head and neck (non-OPSCC).
METHODS: Patients diagnosed with non-OPSCC and known HPV status were identified in the National Cancer Database (NCDB). Multivariate logistic regression was applied to examine factors associated with HPV status. Multivariate analysis was utilized to determine factors correlated with overall survival. Propensity score-weighted Kaplan-Meier estimation was used to adjust for confounders in survival analyses. Multiple imputation method was used for sensitivity analysis.
RESULTS: We identified 19,993 non-OPSCC patients with 5070 being positive for HPV in the NCDB. Median follow-up was 23.5 months. HPV-positive patients were more commonly male, white, with a lower comorbidity index score, presenting with T-stage <2, and N-stage ≥1. Unadjusted 3-year overall survival was 62% and 80% for HPV-negative and HPV-positive patients, respectively (p < 0.0001). On multivariate analysis, mortality was reduced for HPV-positive patients with early stage (HR = 0.68) and locally advanced disease (HR = 0.46). Adjusted 3-year overall survival was 65% for HPV-negative and 76% for HPV-positive patients (p < 0.0001). The survival advantage of HPV was maintained in all subsites and robust on sensitivity analysis.
CONCLUSIONS: Patients with HPV-positive non-OPSCC exhibit similar characteristics as HPV-positive OPSCC. Overall survival was significantly higher for patients with HPV-positive versus HPV-negative non-OPSCC. These data reveal that HPV-positive non-OPSCC represent a favorable cohort that warrants recognition in the design of future clinical trial investigation.
PMID:28752235 | PMC:PMC7069668 | DOI:10.1007/s00432-017-2481-8
View details for PubMedID 28752235
-
More
-
Clinical outcomes for patients presenting with N3 head and neck squamous cell carcinoma: Analysis of the National Cancer Database Head & neck
Ko HC, Chen S, Wieland AM, Yu M, Baschnagel AM, Hartig GK, Harari PM, Witek ME
2017 Nov;39(11):2159-2170. doi: 10.1002/hed.24881. Epub 2017 Jul 24.
-
More
BACKGROUND: There is a paucity of data regarding head and neck squamous cell carcinomas (HNSCCs) and N3 nodal disease.
METHODS: Retrospective analysis of patients with N3 HNSCC identified in the National Cancer Database (NCDB) was performed.
RESULTS: We identified 4867 patients with N3 HNSCC treated with primary surgery or chemoradiotherapy (CRT). Propensity-adjusted median survival was 54.2 and 44.8 months for surgery and CRT, respectively (P = .06). Oropharyngeal primary subsite demonstrated a survival advantage with surgery versus CRT with propensity-adjusted median survivals of 86.0 and 61.9 months, respectively (P < .05).
CONCLUSION: Management of N3 HNSCC relies largely on CRT. Patients with N3 nodal disease with nonoropharyngeal primary tumors exhibit 5-year overall survival approaching 30% independent of initial treatment modality. Patients with oropharyngeal primaries exhibit improved outcomes with surgery largely influenced by the human papillomavirus (HPV)-negative subset. These data represent the most comprehensive analysis of N3 HNSCC outcomes and serves as a foundation for future research and clinical management.
PMID:28737019 | PMC:PMC5647211 | DOI:10.1002/hed.24881
View details for PubMedID 28737019
-
More
-
Small cell carcinoma of the head and neck: An analysis of the National Cancer Database Oral oncology
Pointer KB, Ko HC, Brower JV, Witek ME, Kimple RJ, Lloyd RV, Harari PM, Baschnagel AM
2017 Jun;69:92-98. doi: 10.1016/j.oraloncology.2017.04.009. Epub 2017 Apr 25.
-
More
PURPOSE/OBJECTIVE(S): To evaluate treatment trends and overall survival of patients with small cell carcinoma of the head and neck region.
MATERIALS/METHODS: Patients from 2004 to 2012 were identified from the National Cancer Database. Patient demographics and overall survival were analyzed. Multivariable analysis was used to identify predictors of survival.
RESULTS: Among 347,252 head and neck patients a total of 1042 (0.3%) patients with small cell carcinoma were identified. 17% of patients were diagnosed as stage I/II, 61% as stage III/IVA/IVB and 22% as stage IVC disease. The distribution by anatomic site was 9% oral cavity, 12% oropharynx, 35% larynx, 4% hypopharynx, 10% nasopharynx and 30% nasal cavity and paranasal sinuses. The median overall survival by anatomical site was 20.8months for oral cavity, 23.7months for oropharynx, 17.9months for larynx/hypopharynx, 15.1months for nasopharynx and 36.4months for nasal cavity primary tumors. On multivariable analysis across stage, patients with nasal cavity and paranasal sinuses tumors had the best survival and patients with nasopharynx primaries had the worst survival. In stage I/II patients, type of treatment delivered resulted in no overall survival difference (p=0.78). In patients with locally advanced disease, there was no difference in survival between those treated with combined surgery, radiotherapy and chemotherapy compared to those treated only with radiotherapy and chemotherapy (p=0.46). The addition of radiotherapy to chemotherapy in the metastatic setting did not result in improved survival (p=0.14).
CONCLUSIONS: Small cell carcinoma of the head and neck is a rare malignancy with a poor prognosis. The addition of surgery to radiotherapy and chemotherapy did not improve survival in patients with locally advanced disease.
PMID:28559027 | PMC:PMC5553627 | DOI:10.1016/j.oraloncology.2017.04.009
View details for PubMedID 28559027
-
More
-
In Reply to Fang et al International journal of radiation oncology, biology, physics
Brower JV, Baschnagel AM
2017 Apr 1;97(5):1106-1107. doi: 10.1016/j.ijrobp.2017.01.235. Epub 2017 Mar 15.
-
The significance of Trk receptors in pancreatic cancer Tumour biology : the journal of the International Society for Oncodevelopmental Biology and Medicine
Johnson MD, Stone B, Thibodeau BJ, Baschnagel AM, Galoforo S, Fortier LE, Ketelsen B, Ahmed S, Kelley Z, Hana A, Wilson TG, Robertson JM, Jury RP, Wilson GD
2017 Feb;39(2):1010428317692256. doi: 10.1177/1010428317692256.
-
More
This study investigated the Trk receptor family as a therapeutic target in pancreatic ductal adenocarcinoma and assessed their prognostic significance. Global gene expression analysis was investigated in prospectively collected pancreatic ductal adenocarcinomas that had either undergone neoadjuvant chemoradiation or were treated by surgery. PANC-1 and MIA-PaCa-2 cell lines were investigated to establish whether fractionated radiation altered expression of four neuroendocrine genes and whether this resulted in subsequent changes in radiosensitivity. A specific inhibitor of TrkA, B, and C, AstraZeneca 1332, was investigated in vitro and in vivo in combination with radiation. A tissue microarray was constructed from 77 pancreatic ductal adenocarcinoma patients who had undergone neoadjuvant chemoradiation and the Trk receptor, and neurogenic differentiation 1 expression was assessed and correlated with overall survival. A total of 99 genes were identified that were differentially expressed in the chemoradiation patients with neuroendocrine genes and pathways, in particular the neurogenic differentiation 1 and Trk receptor family, being prominent. Fractionated radiation upregulated the expression of neuroendocrine genes, and AstraZeneca 1332 treatment in vitro enhanced radiosensitivity. No added effect of AstraZeneca 1332 was observed in vivo. Trk receptor expression varied between isoforms but did not correlate significantly with clinical outcome. Radiation treatment upregulated neuroendocrine gene expression but the Trk receptor family does not appear to be a promising treatment target.
PMID:28218045 | DOI:10.1177/1010428317692256
View details for PubMedID 28218045
-
More
-
Dosimetric Comparison of Real-Time MRI-Guided Tri-Cobalt-60 Versus Linear Accelerator-Based Stereotactic Body Radiation Therapy Lung Cancer Plans Technology in cancer research & treatment
Wojcieszynski AP, Hill PM, Rosenberg SA, Hullett CR, Labby ZE, Paliwal B, Geurts MW, Bayliss RA, Bayouth JE, Harari PM, Bassetti MF, Baschnagel AM
2017 Jun;16(3):366-372. doi: 10.1177/1533034617691407. Epub 2017 Feb 7.
-
More
PURPOSE: Magnetic resonance imaging-guided radiation therapy has entered clinical practice at several major treatment centers. Treatment of early-stage non-small cell lung cancer with stereotactic body radiation therapy is one potential application of this modality, as some form of respiratory motion management is important to address. We hypothesize that magnetic resonance imaging-guided tri-cobalt-60 radiation therapy can be used to generate clinically acceptable stereotactic body radiation therapy treatment plans. Here, we report on a dosimetric comparison between magnetic resonance imaging-guided radiation therapy plans and internal target volume-based plans utilizing volumetric-modulated arc therapy.
MATERIALS AND METHODS: Ten patients with early-stage non-small cell lung cancer who underwent radiation therapy planning and treatment were studied. Following 4-dimensional computed tomography, patient images were used to generate clinically deliverable plans. For volumetric-modulated arc therapy plans, the planning tumor volume was defined as an internal target volume + 0.5 cm. For magnetic resonance imaging-guided plans, a single mid-inspiratory cycle was used to define a gross tumor volume, then expanded 0.3 cm to the planning tumor volume. Treatment plan parameters were compared.
RESULTS: Planning tumor volumes trended larger for volumetric-modulated arc therapy-based plans, with a mean planning tumor volume of 47.4 mL versus 24.8 mL for magnetic resonance imaging-guided plans ( P = .08). Clinically acceptable plans were achievable via both methods, with bilateral lung V20, 3.9% versus 4.8% ( P = .62). The volume of chest wall receiving greater than 30 Gy was also similar, 22.1 versus 19.8 mL ( P = .78), as were all other parameters commonly used for lung stereotactic body radiation therapy. The ratio of the 50% isodose volume to planning tumor volume was lower in volumetric-modulated arc therapy plans, 4.19 versus 10.0 ( P < .001). Heterogeneity index was comparable between plans, 1.25 versus 1.25 ( P = .98).
CONCLUSION: Magnetic resonance imaging-guided tri-cobalt-60 radiation therapy is capable of delivering lung high-quality stereotactic body radiation therapy plans that are clinically acceptable as compared to volumetric-modulated arc therapy-based plans. Real-time magnetic resonance imaging provides the unique capacity to directly observe tumor motion during treatment for purposes of motion management.
PMID:28168936 | PMC:PMC5616053 | DOI:10.1177/1533034617691407
View details for PubMedID 28168936
-
More
-
Strategies for Translating Evidence-Based Medicine in Lung Cancer into Community Practice Current oncology reports
Rosenberg SA, Baschnagel AM, Bagley SJ, Housri N
2017 Jan;19(1):5. doi: 10.1007/s11912-017-0563-z.
-
More
The landscape of non-small cell lung cancer (NSCLC) treatment has rapidly evolved over the past decade. This is exemplified by the use of molecular targeted agents, immunotherapies, and newer technologies such as stereotactic body radiotherapy (SBRT). As the translation of preclinical discoveries into clinical practice continues, the effective dissemination and implementation of evidence-based treatment of NSCLC will remain a foremost challenge for oncologists. To further extend evidence-based medicine into the community setting, community oncologists are being engaged on multiple fronts including leadership and participation in national clinical trials and utilization of internet-based resources.
PMID:28168606 | DOI:10.1007/s11912-017-0563-z
View details for PubMedID 28168606
-
More
-
Radiation Dose Escalation in Esophageal Cancer Revisited: A Contemporary Analysis of the National Cancer Data Base, 2004 to 2012 International journal of radiation oncology, biology, physics
Brower JV, Chen S, Bassetti MF, Yu M, Harari PM, Ritter MA, Baschnagel AM
2016 Dec 1;96(5):985-993. doi: 10.1016/j.ijrobp.2016.08.016. Epub 2016 Aug 23.
-
More
PURPOSE: To evaluate the effect of radiation dose escalation on overall survival (OS) for patients with nonmetastatic esophageal cancer treated with concurrent radiation and chemotherapy.
METHODS AND MATERIALS: Patients diagnosed with stage I to III esophageal cancer treated from 2004 to 2012 were identified from the National Cancer Data Base. Patients who received concurrent radiation and chemotherapy with radiation doses of ≥50 Gy and did not undergo surgery were included. OS was compared using Cox proportional hazards regression and propensity score matching.
RESULTS: A total of 6854 patients were included; 3821 (55.7%) received 50 to 50.4 Gy and 3033 (44.3%) received doses >50.4 Gy. Univariate analysis revealed no significant difference in OS between patients receiving 50 to 50.4 Gy and those receiving >50.4 Gy (P=.53). The dose analysis, binned as 50 to 50.4, 51 to 54, 55 to 60, and >60 Gy, revealed no appreciable difference in OS within any group compared with 50 to 50.4 Gy. Subgroup analyses investigating the effect of dose escalation by histologic type and in the setting of intensity modulated radiation therapy also failed to reveal a benefit. Propensity score matching confirmed the absence of a statistically significant difference in OS among the dose levels. The factors associated with improved OS on multivariable analysis included female sex, lower Charlson-Deyo comorbidity score, private insurance, cervical/upper esophagus location, squamous cell histologic type, lower T stage, and node-negative status (P<.01 for all analyses).
CONCLUSIONS: In this large national cohort, dose escalation >50.4 Gy did not result in improved OS among patients with stage I to III esophageal cancer treated with definitive concurrent radiation and chemotherapy. These data suggest that despite advanced contemporary treatment techniques, OS for patients with esophageal cancer remains unaltered by escalation of radiation dose >50.4 Gy, consistent with the results of the INT-0123 trial. Furthermore, these data highlight that many radiation oncologists have not embraced the concept that dose escalation does not improve OS. Although local control, not investigated in the present study, might benefit from dose escalation, novel therapies are needed to improve the OS of patients with esophageal cancer.
PMID:27869098 | DOI:10.1016/j.ijrobp.2016.08.016
View details for PubMedID 27869098
-
More
-
Combined CD44, c-MET, and EGFR expression in p16-positive and p16-negative head and neck squamous cell carcinomas Journal of oral pathology & medicine : official publication of the International Association of Oral Pathologists and the American Academy of Oral Pathology
Baschnagel AM, Tonlaar N, Eskandari M, Kumar T, Williams L, Hanna A, Pruetz BL, Wilson GD
2017 Mar;46(3):208-213. doi: 10.1111/jop.12478. Epub 2016 Jul 21.
-
More
PURPOSE/OBJECTIVE(S): To examine the association between CD44 and c-MET expression in relation to p16 and EGFR in patients with head and neck squamous cell carcinoma (HNSCC).
MATERIALS/METHODS: Immunohistochemical staining of CD44, p16, EGFR, and c-MET was performed on 105 locally advanced HNSCC patients treated with chemoradiation. CD44 expression was correlated with c-MET, EGFR, and p16, locoregional control (LRC), distant metastases (DM), disease-free survival (DFS) and overall survival (OS).
RESULTS: High CD44 expression was present in 33% of patients and was associated with non-oropharynx primaries (P < 0.001), high c-MET expression (P < 0.001), p16-negative (P < 0.001) and EGFR-positive tumors (P < 0.001). Fifty-seven percent of CD44 high expressing tumors had high c-MET expression compared to 21% of CD44 low expressing tumors (P < 0.001). High CD44 expression predicted for worse LRC (HR: 2.44; 95% CI: 1.16-5.13; P = 0.018), DFS (HR: 2.61; 95% CI: 1.46-4.67; P = 0.001), and OS (HR: 2.52; 95% CI: 1.30-4.92; P = 0.007) but not DM (P = 0.57) on univariate analysis. Patients with both high CD44 and c-MET expression had a poor prognosis with a 2-year DFS of 30% compared to 70% in the rest of the cohort (P = 0.003). On multivariable analysis, after adjusting for site, T-stage, smoking history, and EGFR status, high c-MET (P = 0.039) and negative p16 status (P = 0.034) predicted for worse DFS, while high CD44 expression did not (P = 0.43).
CONCLUSIONS: High CD44 expression is associated with high c-MET expression, p16-negative tumors, and EGFR-positive tumors. The combination of these markers predicts for poor prognosis in HNSCC patients treated with chemoradiation.
PMID:27442811 | DOI:10.1111/jop.12478
View details for PubMedID 27442811
-
More
-
Cancer Stem Cell Signaling during Repopulation in Head and Neck Cancer Stem cells international
Wilson GD, Thibodeau BJ, Fortier LE, Pruetz BL, Galoforo S, Marples B, Baschnagel AM, Akervall J, Huang J
2016;2016:1894782. doi: 10.1155/2016/1894782. Epub 2016 Jan 6.
-
More
The aim of the study was to investigate cancer stem signaling during the repopulation response of a head and neck squamous cell cancer (HNSCC) xenograft after radiation treatment. Xenografts were generated from low passage HNSCC cells and were treated with either sham radiation or 15 Gy in one fraction. At different time points, days 0, 3, and 10 for controls and days 4, 7, 12, and 21, after irradiation, 3 tumors per group were harvested for global gene expression, pathway analysis, and immunohistochemical evaluation. 316 genes were identified that were associated with a series of stem cell-related genes and were differentially expressed (p ≤ 0.01 and 1.5-fold) at a minimum of one time point in UT-SCC-14 xenografts after radiation. The largest network of genes that showed significant changes after irradiation was associated with CD44, NOTCH1, and MET. c-MET and ALDH1A3 staining correlated with the changes in gene expression. A clear pattern emerged that was consistent with the growth inhibition data in that genes associated with stem cell pathways were most active at day 7 and day 12 after irradiation. The MET/CD44 axis seemed to be an important component of the repopulation response.
PMID:26880935 | PMC:PMC4736761 | DOI:10.1155/2016/1894782
View details for PubMedID 26880935
-
More
-
Surgical Resection of Brain Metastases and the Risk of Leptomeningeal Recurrence in Patients Treated With Stereotactic Radiosurgery International journal of radiation oncology, biology, physics
Johnson MD, Avkshtol V, Baschnagel AM, Meyer K, Ye H, Grills IS, Chen PY, Maitz A, Olson RE, Pieper DR, Krauss DJ
2016 Mar 1;94(3):537-43. doi: 10.1016/j.ijrobp.2015.11.022. Epub 2015 Nov 19.
-
More
PURPOSE: Recent prospective data have shown that patients with solitary or oligometastatic disease to the brain may be treated with upfront stereotactic radiosurgery (SRS) with deferral of whole-brain radiation therapy (WBRT). This has been extrapolated to the treatment of patients with resected lesions. The aim of this study was to assess the risk of leptomeningeal disease (LMD) in patients treated with SRS to the postsurgical resection cavity for brain metastases compared with patients treated with SRS to intact metastases.
METHODS AND MATERIALS: Four hundred sixty-five patients treated with SRS without upfront WBRT at a single institution were identified; 330 of these with at least 3 months' follow-up were included in this analysis. One hundred twelve patients had undergone surgical resection of at least 1 lesion before SRS compared with 218 treated for intact metastases. Time to LMD and overall survival (OS) time were estimated from date of radiosurgery, and LMD was analyzed by the use of cumulative incidence method with death as a competing risk. Univariate and multivariate analyses were performed with competing risk regression to determine whether various clinical factors predicted for LMD.
RESULTS: With a median follow-up time of 9.0 months, 39 patients (12%) experienced LMD at a median of 6.0 months after SRS. At 1 year, the cumulative incidence of LMD, with death as a competing risk, was 5.2% for the patients without surgical resection versus 16.9% for those treated with surgery (Gray test, P<.01). On multivariate analysis, prior surgical resection (P<.01) and breast cancer primary (P=.03) were significant predictors of LMD development. The median OS times for patients undergoing surgery compared with SRS alone were 12.9 and 10.6 months, respectively (log-rank P=.06).
CONCLUSIONS: In patients undergoing SRS with deferral of upfront WBRT for intracranial metastatic disease, prior surgical resection and breast cancer primary are associated with an increased risk for the development of LMD.
PMID:26867883 | DOI:10.1016/j.ijrobp.2015.11.022
View details for PubMedID 26867883
-
More
-
Crizotinib Fails to Enhance the Effect of Radiation in Head and Neck Squamous Cell Carcinoma Xenografts Anticancer research
Baschnagel AM, Galoforo S, Thibodeau BJ, Ahmed S, Nirmal S, Akervall J, Wilson GD
2015 Nov;35(11):5973-82.
-
More
AIM: Mesenchymal-epithelial transition factor (MET), a receptor tyrosine kinase, is expressed in head and neck squamous cell carcinomas (HNSCC) and is involved in tumor progression and associated with poor prognosis. MET can be inhibited by crizotinib, a potent ATP-competitive kinase inhibitor. We examined the effects of combining crizotinib and radiation in a pre-clinical HNSCC model.
MATERIALS AND METHODS: Nine HNSCC cell lines were screened for MET expression, copy-number amplification and mutational status. The in vitro effects of crizotinib and radiation were assessed with clonogenic survival assays. MET signaling proteins were assessed with western blot and receptor tyrosine kinase array. Tumor growth-delay experiments with UT-SCC-14 and UT-SCC-15 oral tongue xenografts were used to assess in vivo tumor radiosensitivity.
RESULTS: All nine HNSCC cell lines showed a varying degree of MET protein and RNA expression. Increased MET copy number was not present. MET was expressed after irradiation both in vitro and in vivo. Crizotinib alone inhibited phosphorylation of MET and inhibited cell growth in vitro but did not inhibit phosphorylation of downstream signaling proteins: MAPK, AKT or c-SRC. When combined with radiation in vitro, crizotinib demonstrated radiation enhancement in only one cell line. Crizotinib did not enhance the effect of radiation in either UT-SCC-14 or UT-SCC-15 tumors grown as xenografts.
CONCLUSION: MET is overexpressed in HNSCC cell lines, however, crizotinib failed to enhance the radiation response and failed to inhibit MET downstream signaling proteins in this HNSCC model.
PMID:26504020
View details for PubMedID 26504020
-
More
-
The association of (18)F-FDG PET and glucose metabolism biomarkers GLUT1 and HK2 in p16 positive and negative head and neck squamous cell carcinomas Radiotherapy and oncology : journal of the European Society for Therapeutic Radiology and Oncology
Baschnagel AM, Wobb JL, Dilworth JT, Williams L, Eskandari M, Wu D, Pruetz BL, Wilson GD
2015 Oct;117(1):118-24. doi: 10.1016/j.radonc.2015.08.025. Epub 2015 Aug 29.
-
More
OBJECTIVES: To investigate the relationship between FDG-PET maximum standard uptake value (SUVmax), p16, EGFR, GLUT1 and HK2 expression in head and neck squamous cell carcinomas (HNSCC).
MATERIALS AND METHODS: Immunohistochemical staining of p16, EGFR, GLUT1 and HK2 was performed on primary tumor tissue from 97 locally advanced HNSCC patients treated with definitive chemoradiation. SUVmax along with p16, EGFR, GLUT1 and HK2 expression were analyzed for associations including local control, locoregional control and disease free survival.
RESULTS: Pretreatment SUVmax in primary tumors did not differ when stratified by p16, EGFR or GLUT1 expression but SUVmax was significantly higher in HK2 expressing tumors (p=0.021) and in tumors with higher T-stage (p=0.022). GLUT1 expression was significantly higher in p16 negative (p<0.001) and EGFR positive tumors (p<0.01). HK2 expressing tumors were associated with EGFR positive tumors (p=0.022) but not with p16 or GLUT1 expression. EGFR positive, p16 negative and high GLUT1 expressing tumors were associated with worse local control and disease free survival on univariate analyses. After adjusting for patient and treatment characteristics p16 status was the only factor that predicted for outcome on multivariate analysis.
CONCLUSIONS: High GLUT1 expression was associated with EGFR positive and p16 negative HNSCC tumors. GLUT1 maybe an important biomarker in HNSCC but its expression appears dependent on p16 status.
PMID:26328941 | DOI:10.1016/j.radonc.2015.08.025
View details for PubMedID 26328941
-
More
-
Glucose metabolism gene expression patterns and tumor uptake of ¹⁸F-fluorodeoxyglucose after radiation treatment International journal of radiation oncology, biology, physics
Wilson GD, Thibodeau BJ, Fortier LE, Pruetz BL, Galoforo S, Baschnagel AM, Chunta J, Wong YO, Yan D, Marples B, Huang J
2014 Nov 1;90(3):620-7. doi: 10.1016/j.ijrobp.2014.06.062. Epub 2014 Sep 26.
-
More
PURPOSE: To investigate whether radiation treatment influences the expression of glucose metabolism genes and compromises the potential use of (18)F-fluorodeoxyglucose positron emission tomography (FDG-PET) as a tool to monitor the early response of head and neck cancer xenografts to radiation therapy (RT).
METHODS AND MATERIALS: Low passage head and neck squamous cancer cells (UT14) were injected to the flanks of female nu/nu mice to generate xenografts. After tumors reached a size of 500 mm(3) they were treated with either sham RT or 15 Gy in 1 fraction. At different time points, days 3, 9, and 16 for controls and days 4, 7, 12, 21, 30, and 40 after irradiation, 2 to 3 mice were assessed with dynamic FDG-PET acquisition over 2 hours. Immediately after the FDG-PET the tumors were harvested for global gene expression analysis and immunohistochemical evaluation of GLUT1 and HK2. Different analytic parameters were used to process the dynamic PET data.
RESULTS: Radiation had no effect on key genes involved in FDG uptake and metabolism but did alter other genes in the HIF1α and glucose transport-related pathways. In contrast to the lack of effect on gene expression, changes in the protein expression patterns of the key genes GLUT1/SLC2A1 and HK2 were observed after radiation treatment. The changes in GLUT1 protein expression showed some correlation with dynamic FDG-PET parameters, such as the kinetic index.
CONCLUSION: (18)F-fluorodeoxyglucose positron emission tomography changes after RT would seem to represent an altered metabolic state and not a direct effect on the key genes regulating FDG uptake and metabolism.
PMID:25304950 | DOI:10.1016/j.ijrobp.2014.06.062
View details for PubMedID 25304950
-
More
-
c-Met expression is a marker of poor prognosis in patients with locally advanced head and neck squamous cell carcinoma treated with chemoradiation International journal of radiation oncology, biology, physics
Baschnagel AM, Williams L, Hanna A, Chen PY, Krauss DJ, Pruetz BL, Akervall J, Wilson GD
2014 Mar 1;88(3):701-7. doi: 10.1016/j.ijrobp.2013.11.013.
-
More
PURPOSE: To examine the prognostic significance of c-Met expression in relation to p16 and epidermal growth factor receptor (EGFR) in patients with locally advanced head and neck squamous cell carcinoma (HNSCC) treated with definitive concurrent chemoradiation.
METHODS AND MATERIALS: Archival tissue from 107 HNSCC patients treated with chemoradiation was retrieved, and a tissue microarray was assembled. Immunohistochemical staining of c-Met, p16, and EGFR was performed. c-Met expression was correlated with p16, EGFR, clinical characteristics, and clinical endpoints including locoregional control (LRC), distant metastasis (DM), disease-free survival (DFS), and overall survival (OS).
RESULTS: Fifty-one percent of patients were positive for p16, and 53% were positive for EGFR. Both p16-negative (P≤.001) and EGFR-positive (P=.019) status predicted for worse DFS. Ninety-three percent of patients stained positive for c-Met. Patients were divided into low (0, 1, or 2+ intensity) or high (3+ intensity) c-Met expression. On univariate analysis, high c-Met expression predicted for worse LRC (hazard ratio [HR] 2.27; 95% CI, 1.08-4.77; P=.031), DM (HR 4.41; 95% CI, 1.56-12.45; P=.005), DFS (HR 3.00; 95% CI, 1.68-5.38; P<.001), and OS (HR 4.35; 95% CI, 2.13-8.88; P<.001). On multivariate analysis, after adjustment for site, T stage, smoking history, and EGFR status, only high c-Met expression (P=.011) and negative p16 status (P=.003) predicted for worse DFS. High c-Met expression was predictive of worse DFS in both EGFR-positive (P=.032) and -negative (P=.008) patients. In the p16-negative patients, those with high c-Met expression had worse DFS (P=.036) than did those with low c-Met expression. c-Met expression was not associated with any outcome in the p16-positive patients.
CONCLUSIONS: c-Met is expressed in the majority of locally advanced HNSCC cases, and high c-Met expression predicts for worse clinical outcomes. High c-Met expression predicted for worse DFS in p16-negative patients but not in p16-positive patients. c-Met predicted for worse outcome regardless of EGFR status.
PMID:24521684 | DOI:10.1016/j.ijrobp.2013.11.013
View details for PubMedID 24521684
-
More
-
Trigeminal neuralgia pain relief after gamma knife stereotactic radiosurgery Clinical neurology and neurosurgery
Baschnagel AM, Cartier JL, Dreyer J, Chen PY, Pieper DR, Olson RE, Krauss DJ, Maitz AH, Grills IS
2014 Feb;117:107-111. doi: 10.1016/j.clineuro.2013.12.003. Epub 2013 Dec 15.
-
More
OBJECTIVES: To report outcomes of patients with medical and/or surgical refractory trigeminal neuralgia (TN) treated with gamma knife stereotactic radiosurgery (GK SRS).
METHODS: One hundred and forty-nine patients with 152 cases of TN treated with GK SRS were analyzed. All patients, except one, received a dose of 40Gy to the 50% isodose volume. The Barrow Neurological Institute (BNI) pain intensity score was used to grade pain. Actuarial rates of pain relief were calculated. Multiple factors were analyzed for association with pain relief.
RESULTS: The median follow up was 27 months (4-71 months). Overall 92% of cases achieved a BNI score I-III after GK SRS. Of those who had pain relief after GK SRS, 32% developed pain recurrence defined as a BNI score of IV or V. The actuarial rate of freedom from pain recurrence (BNI scores I-III) of all treated cases at 1, 2 and 3-years was 76%, 69% and 60%, respectively. On univariate analysis age ≥70 was predictive of better pain relief (p=0.046). Type of pain, prior surgery, multiple sclerosis, number of isocenters, treated nerve length, volume and thickness and distance from the root entry zone to the isocenter were not significant for maintaining a BNI score of I-III. Those who achieved a BNI score of I or II were more likely to maintain pain relief compared to those who only achieved a BNI score of III (93% vs 38% at three years, p<0.01). The rate of pain relief of twenty-seven patients who underwent repeat GK SRS was 70% and 62% at 1 and 2 years, respectively. Toxicity after first GK SRS was minimal with 25% of cases experiencing only new or worsening post-treatment numbness.
CONCLUSION: GK SRS provides acceptable pain relief with limited morbidity in patients with medical and/or surgical refractory TN.
PMID:24438815 | DOI:10.1016/j.clineuro.2013.12.003
View details for PubMedID 24438815
-
More
-
Tumor volume as a predictor of survival and local control in patients with brain metastases treated with Gamma Knife surgery Journal of neurosurgery
Baschnagel AM, Meyer KD, Chen PY, Krauss DJ, Olson RE, Pieper DR, Maitz AH, Ye H, Grills IS
2013 Nov;119(5):1139-44. doi: 10.3171/2013.7.JNS13431. Epub 2013 Aug 23.
-
More
OBJECT: The aim of this study was to examine tumor volume as a prognostic factor for patients with brain metastases treated with Gamma Knife surgery (GKS).
METHODS: Two hundred fifty patients with 1-14 brain metastases who had initially undergone GKS alone at a single institution were retrospectively reviewed. Patients who received upfront whole brain radiation therapy were excluded. Survival times were estimated using the Kaplan-Meier method. Univariate and multivariate analyses using Cox proportional hazard regression models were used to determine if various prognostic factors could predict overall survival, distant brain failure, and local control.
RESULTS: Median overall survival was 7.1 months and the 1-year local control rate was 91.5%. Median time to distant brain failure was 8.0 months. On univariate analysis an increasing total tumor volume was significantly associated with worse survival (p = 0.031) whereas the number of brain metastases, analyzed as a continuous variable, was not (p = 0.082). After adjusting for age, Karnofsky Performance Scale score, and extracranial disease on multivariate analysis, total tumor volume was found to be a better predictor of overall survival (p = 0.046) than number of brain metastases analyzed as a continuous variable (p = 0.098). A total tumor volume cutoff value of ≥ 2 cm(3) (p = 0.008) was a stronger predictor of overall survival than the number of brain metastases (p = 0.098). Larger tumor volume and extracranial disease, but not the number of brain metastases, were predictive of distant brain failure on multivariate analysis. Local tumor control at 1 year was 97% for lesions < 2 cm(3) compared with 75% for lesions ≥ 2 cm(3) (p < 0.001).
CONCLUSIONS: After adjusting for other factors, a total brain metastasis volume was a strong and independent predictor for overall survival, distant brain failure, and local control, even when considering the number of metastases.
PMID:23971958 | DOI:10.3171/2013.7.JNS13431
View details for PubMedID 23971958
-
More
-
Toxicities and costs of placing prophylactic and reactive percutaneous gastrostomy tubes in patients with locally advanced head and neck cancers treated with chemoradiotherapy Head & neck
Baschnagel AM, Yadav S, Marina O, Parzuchowski A, Lanni TB, Warner JN, Parzuchowski JS, Ignatius RT, Akervall J, Chen PY, Krauss DJ
2014 Aug;36(8):1155-61. doi: 10.1002/hed.23426. Epub 2013 Nov 27.
-
More
BACKGROUND: We compared dependence rates, complications, toxicities, and costs associated with prophylactic versus reactive percutaneous endoscopic gastrostomy (PEG) tube placement.
METHODS: One hundred ninety-three patients with locally advanced head and neck squamous cell carcinoma treated with concurrent chemoradiotherapy were retrospectively reviewed.
RESULTS: The 1-year and 2-year actuarial PEG tube dependence rate of the entire cohort was 24% and 13%, respectively. There was no difference in the PEG tube dependence rates between those placed prophylactically versus reactively. Patients who received a PEG tube reactively had a significantly higher stricture rate (p = .03) and aspiration rate (p < .001) compared to the prophylactic group. There were significantly fewer hospitalizations in the prophylactic group compared to the reactive group (p = .003). When accounting for both PEG placement and hospitalizations, the prophylactic approach was found to be more cost effective.
CONCLUSION: PEG tubes placed prophylactically were associated with lower rates of strictures, aspirations, hospitalizations, and costs compared to those placed reactively.
PMID:23852670 | DOI:10.1002/hed.23426
View details for PubMedID 23852670
-
More
-
A matched-pair comparison of intensity-modulated radiation therapy with cetuximab versus intensity-modulated radiation therapy with platinum-based chemotherapy for locally advanced head neck cancer International journal of clinical oncology
Huang J, Baschnagel AM, Chen P, Gustafson G, Jaiyesmi I, Folbe M, Ye H, Akervall J, Krauss D
2014 Apr;19(2):240-6. doi: 10.1007/s10147-013-0540-y. Epub 2013 Mar 12.
-
More
PURPOSE: We retrospectively compared the efficacy of intensity-modulated radiotherapy (IMRT) and cetuximab (IMRT/cetuximab) versus IMRT and platinum-based chemotherapy (IMRT/platinum) for locally advanced head neck squamous cell carcinoma (LAHNSCC).
METHODS: Thirty-one IMRT/cetuximab patients were matched 1:2 with 62 IMRT/platinum patients according to primary site and clinical stage. The primary endpoint was locoregional recurrence (LRR), and secondary endpoints included distant metastasis (DM), cause-specific survival (CSS), and overall survival (OS).
RESULTS: Because of inherent selection bias, the IMRT/cetuximab cohort was significantly older and with a higher Charlson Comorbidity Index. IMRT/cetuximab and IMRT/platinum did not have significantly different LRR and DM (33 vs. 23 % at 2 years, P = 0.22; 17 vs. 11 % at 2 years, P = 0.40; respectively). IMRT/cetuximab had significantly worse CSS and OS (67 vs. 84 %, P = 0.04; 58 vs. 83 %, P = 0.001; respectively). However, for the subset of elderly patients ≥65 years old, there is no difference between the two cohorts for all endpoints (all P = NS).
CONCLUSION: IMRT/platinum should remain the preferred choice of chemoradiotherapy for LAHNSCC, but IMRT/cetuximab may be a reasonable alternative for elderly patients.
PMID:23479120 | DOI:10.1007/s10147-013-0540-y
View details for PubMedID 23479120
-
More
-
Hearing preservation in patients with vestibular schwannoma treated with Gamma Knife surgery Journal of neurosurgery
Baschnagel AM, Chen PY, Bojrab D, Pieper D, Kartush J, Didyuk O, Naumann IC, Maitz A, Grills IS
2013 Mar;118(3):571-8. doi: 10.3171/2012.10.JNS12880. Epub 2012 Dec 7.
-
More
OBJECT: Hearing loss after Gamma Knife surgery (GKS) in patients with vestibular schwannoma has been associated with radiation dose to the cochlea. The purpose of this study was to evaluate serviceable hearing preservation in patients with VS who were treated with GKS and to determine if serviceable hearing loss can be correlated with the dose to the cochlea.
METHODS: Forty patients with vestibular schwannoma with serviceable hearing were treated using GKS with a median marginal dose of 12.5 Gy (range 12.5-13 Gy) to the 50% isodose volume. Audiometry was performed prospectively before and after GKS at 1, 3, and 6 months, and then every 6 months thereafter. Hearing preservation was based on pure tone average (PTA) and speech discrimination (SD). Serviceable hearing was defined as PTA less than 50 dB and SD greater than 50%.
RESULTS: The median cochlear maximum and mean doses were 6.9 Gy (range 1.6-16 Gy) and 2.7 Gy (range 0.7-5.0 Gy), respectively. With a median audiological follow-up of 35 months (range 6-58 months), the 1-, 2-, and 3-year actuarial rates of maintaining serviceable hearing were 93%, 77%, and 74%, respectively. No patient who received a mean cochlear dose less than 2 Gy experienced serviceable hearing loss (p = 0.035). Patients who received a mean cochlear dose less than 3 Gy had a 2-year hearing preservation rate of 91% compared with 59% in those who received a mean cochlear dose of 3 Gy or greater (p = 0.029). Those who had more than 25% of their cochlea receiving 3 Gy or greater had a higher rate of hearing loss (p = 0.030). There was no statistically significant correlation between serviceable hearing loss and age, tumor size, pre-GKS PTA, pre-GKS SD, pre-GKS Gardner-Robertson class, maximum cochlear dose, or the percentage of cochlear volume receiving 5 Gy. On multivariate analysis there was a trend toward significance for serviceable hearing loss with a mean cochlear dose of 3 Gy or greater (p = 0.074). Local control was 100% at 24 months. No patient developed facial or trigeminal nerve dysfunction.
CONCLUSIONS: With a median mean cochlear dose of 2.7 Gy, the majority of patients with serviceable hearing retained serviceable hearing 3 years after GKS. A mean cochlear dose less than 3 Gy was associated with higher serviceable hearing preservation.
PMID:23216466 | DOI:10.3171/2012.10.JNS12880
View details for PubMedID 23216466
-
More
-
Failure rate and cosmesis of immediate tissue expander/implant breast reconstruction after postmastectomy irradiation Clinical breast cancer
Baschnagel AM, Shah C, Wilkinson JB, Dekhne N, Arthur DW, Vicini FA
2012 Dec;12(6):428-32. doi: 10.1016/j.clbc.2012.09.001. Epub 2012 Oct 11.
-
More
BACKGROUND: This study reports the rate of breast reconstruction failure and cosmetic outcomes after postmastectomy radiation therapy (PMRT) with temporary tissue expanders (TEs) or implants in place.
PATIENTS AND METHODS: Ninety-four patients underwent mastectomy (93 unilateral, 1 bilateral; 95 cases total) and immediate TE reconstruction followed by PMRT. Ninety TEs and 5 permanent implants were irradiated. All patients received a dose of 5400 cGy given in 180-cGy fractions to the reconstructed breast. Twenty-one patients (22%) received tangents alone and 74 patients (78%) were treated with tangents and a supraclavicular field using a monoisocentric technique. Bolus was used in 91 patients (96%). Eighty-eight patients (93%) received chemotherapy and 78 patients (82%) received endocrine therapy.
RESULTS: With a median follow-up of 24.1 months, 19 patients (20%) experienced failure of reconstruction. The 1-, 2-, and 3-year actuarial rate of reconstruction failure was 9.7%, 19.3%, and 25.5%, respectively. Infection was the most common cause of failure. Of the 19 failures, 8 patients underwent salvage procedures with flap reconstruction. Univariate analysis was performed examining age, chemotherapy use, hormone therapy use, use of a supraclavicular field, smoking status, diabetes, hypertension, and menopausal status. No risk factors were found to be associated with reconstruction failure. In patients who did not experience reconstruction failure, good/excellent cosmesis was observed in 75% of patients.
CONCLUSION: In the current series of women with a high risk of locoregional recurrence, PMRT with a TE/implant in place provides good cosmesis in the majority of women, with an acceptable risk of expander or implant loss.
PMID:23062707 | DOI:10.1016/j.clbc.2012.09.001
View details for PubMedID 23062707
-
More
-
Survival after chemoradiation in resected pancreatic cancer: the impact of adjuvant gemcitabine International journal of radiation oncology, biology, physics
Baschnagel A, Shah C, Margolis J, Nadeau L, Stein J, Jury R, Robertson JM
2012 Jul 1;83(3):e331-5. doi: 10.1016/j.ijrobp.2012.01.008. Epub 2012 Mar 13.
-
More
PURPOSE: To evaluate survival in patients with resected pancreatic cancer treated with concurrent chemoradiation with or without adjuvant gemcitabine (Gem).
METHODS AND MATERIALS: From 1998 to 2010, 86 patients with pancreatic adenocarcinoma who underwent resection were treated with adjuvant concurrent chemoradiation. Thirty-four patients received concurrent 5-fluorouracil-based chemoradiation (5-FU/RT) with traditional field radiation (range, 45-61.2 Gy; median, 50.4 Gy) without further adjuvant therapy. Thirty patients received traditional field 5-FU/RT (range, 45-60.4 Gy; median, 50.4 Gy) with Gem (1,000 mg/m(2) weekly) either before and after radiotherapy or only after radiotherapy. Twenty-two patients received concurrent full-dose Gem (1,000 mg/m(2) weekly)-based chemoradiation (Gem/RT), consisting of involved-field radiation (range, 27-38 Gy; median, 36 Gy) followed by further adjuvant Gem.
RESULTS: The median age of the cohort was 65 years (range, 40-80 years). Of the patients, 58 had T3 tumors (67%), 22 had T2 tumors (26%), and 6 had T1 tumors (7%). N1 disease was present in 61 patients (71%), whereas 18 patients (21%) had R1 resections. Performance status, lymph node status, and margin status were all similar among the treatment groups. Median follow-up was 19.0 months. Median overall survival (OS) (19.2 months, 19.0 months, and 21.0 months) and 3-year OS rates (26.5%, 27.2%, and 32.1%) were similar among patients with 5-FU/RT with no adjuvant Gem, those with 5-FU/RT with adjuvant Gem, and those with Gem/RT with adjuvant Gem, respectively (p = 0.88). Patients who received adjuvant Gem had a similar median OS (22.1 months) and 3-year OS rate (29%) compared to patients who did not (19.2 months and 26.5%, respectively) (p = 0.62). There was a trend for improved 3-year OS rates in patients with R0 vs. R1 resections (28.1% vs. 14.2%, p = 0.06) and in patients with T1 and T2 vs. T3 tumors (38% vs. 20%, p = 0.09). Node-negative patients had an improved 3-year OS rate (30.1%) when compared with patients with N1 disease (16.2%) (p = 0.02).
CONCLUSION: In our cohort of patients with resected pancreatic cancer, Gem chemotherapy did not improve OS after chemoradiotherapy.
PMID:22420967 | DOI:10.1016/j.ijrobp.2012.01.008
View details for PubMedID 22420967
-
More
-
Factors associated with the development of breast cancer-related lymphedema after whole-breast irradiation International journal of radiation oncology, biology, physics
Shah C, Wilkinson JB, Baschnagel A, Ghilezan M, Riutta J, Dekhne N, Balaraman S, Mitchell C, Wallace M, Vicini F
2012 Jul 15;83(4):1095-100. doi: 10.1016/j.ijrobp.2011.09.058. Epub 2011 Nov 16.
-
More
PURPOSE: To determine the rates of breast cancer-related lymphedema (BCRL) in patients undergoing whole-breast irradiation as part of breast-conserving therapy (BCT) and to identify clinical, pathologic, and treatment factors associated with its development.
METHODS AND MATERIALS: A total of 1,861 patients with breast cancer were treated at William Beaumont Hospital with whole-breast irradiation as part of their BCT from January 1980 to February 2006, with 1,497 patients available for analysis. Determination of BCRL was based on clinical assessment. Differences in clinical, pathologic, and treatment characteristics between patients with BCRL and those without BCRL were evaluated, and the actuarial rates of BCRL by regional irradiation technique were determined.
RESULTS: The actuarial rate of any BCRL was 7.4% for the entire cohort and 9.9%, 14.7%, and 8.3% for patients receiving a supraclavicular field, posterior axillary boost, and internal mammary irradiation, respectively. BCRL was more likely to develop in patients with advanced nodal status (11.4% vs. 6.3%, p = 0.001), those who had a greater number of lymph nodes removed (14 nodes) (9.5% vs. 6.0%, p = 0.01), those who had extracapsular extension (13.4% vs. 6.9%, p = 0.009), those with Grade II/III disease (10.8% vs. 2.9%, p < 0.001), and those who received adjuvant chemotherapy (10.5% vs. 6.7%, p = 0.02). Regional irradiation showed small increases in the rates of BCRL (p = not significant).
CONCLUSIONS: These results suggest that clinically detectable BCRL will develop after traditional BCT in up to 10% of patients. High-risk subgroups include patients with advanced nodal status, those with more nodes removed, and those who receive chemotherapy, with patients receiving regional irradiation showing a trend toward increased rates.
PMID:22099041 | DOI:10.1016/j.ijrobp.2011.09.058
View details for PubMedID 22099041
-
More
-
Long-term quality of life after radiotherapy for the treatment of anal cancer Cancer
Das P, Cantor SB, Parker CL, Zampieri JB, Baschnagel A, Eng C, Delclos ME, Krishnan S, Janjan NA, Crane CH
2010 Feb 15;116(4):822-9. doi: 10.1002/cncr.24906.
-
More
BACKGROUND: Radiotherapy is the current standard of care for patients with localized squamous cell cancer of the anal canal. The goal of the current study was to evaluate long-term quality of life (QoL) in patients after this treatment.
METHODS: Questionnaires were mailed to 80 patients treated with definitive radiotherapy, with or without concurrent chemotherapy, for anal cancer, with a minimum 2-year interval after the completion of radiotherapy. The questionnaire included the Functional Assessment of Cancer Therapy-Colorectal (FACT-C), the Medical Outcomes Study (MOS) Sexual Problems Scale, and questions regarding demographic characteristics and comorbidities.
RESULTS: A total of 32 (40%) patients completed the questionnaire. There were no significant differences noted with regard to clinical and demographic characteristics between the survey responders and nonresponders. Among the 32 responders, the median dose of radiotherapy was 55 Grays (Gy), and 97% had received concurrent chemotherapy. The median interval between radiotherapy and survey participation was 5 years (range, 3-13 years). The median total FACT-C score was 108 (range, 47-128), of a maximum (best possible) score of 136. Patients who reported depression or anxiety and younger patients were found to have significantly lower total FACT-C scores. The median scores on the Physical, Social/Family, Emotional, Functional, and Colorectal subscales of the FACT-C were 20, 23, 21, 22, and 21, respectively, of maximum (best possible) scores of 28, 28, 24, 28, and 28, respectively. The median score on the MOS Sexual Problems Scale was 67 (range, 0-100), of a maximum (worst possible) score of 100.
CONCLUSIONS: Patients treated with radiotherapy for anal cancer reported acceptable overall QoL scores, but poor sexual function scores. Investigations are warranted into more modern radiation techniques that could potentially reduce late toxicity from radiotherapy.
PMID:20041481 | PMC:PMC6455911 | DOI:10.1002/cncr.24906
View details for PubMedID 20041481
-
More
-
Vorinostat enhances the radiosensitivity of a breast cancer brain metastatic cell line grown in vitro and as intracranial xenografts Molecular cancer therapeutics
Baschnagel A, Russo A, Burgan WE, Carter D, Beam K, Palmieri D, Steeg PS, Tofilon P, Camphausen K
2009 Jun;8(6):1589-95. doi: 10.1158/1535-7163.MCT-09-0038. Epub 2009 Jun 9.
-
More
Vorinostat (suberoylanilide hydroxamic acid), a histone deacetylase inhibitor, is currently undergoing clinical evaluation as therapy for cancer. We investigated the effects of vorinostat on tumor cell radiosensitivity in a breast cancer brain metastasis model using MDA-MB-231-BR cells. In vitro radiosensitivity was evaluated using clonogenic assay. Cell cycle distribution and apoptosis was measured using flow cytometry. DNA damage and repair was evaluated using gammaH2AX. Mitotic catastrophe was measured by immunostaining. Growth delay and intracranial xenograft models were used to evaluate the in vivo tumor radiosensitivity. Cells exposed to vorinostat for 16 hours before and maintained in the medium after irradiation had an increase in radiosensitivity with a dose enhancement factor of 1.57. gammaH2AX, as an indicator of double-strand breaks, had significantly more foci per cell in the vorinostat plus irradiation group. Mitotic catastrophe, measured at 72 hours, was significantly increased in cells receiving vorinostat plus irradiation. Irradiation of s.c. MDA-MB-231-BR tumors in mice treated with vorinostat resulted in an increase in radiation-induced tumor growth delay. Most importantly, animals with intracranial tumor implants lived the longest after combination treatment. These results indicate that vorinostat enhances tumor cell radiosensitivity in vitro and in vivo. There was a greater than additive improvement in survival in our intracranial model. Combining vorinostat with radiation may be a potential treatment option for patients with breast cancer who develop brain metastases.
PMID:19509253 | PMC:PMC3393105 | DOI:10.1158/1535-7163.MCT-09-0038
View details for PubMedID 19509253
-
More
-
Neuropsychological testing and biomarkers in the management of brain metastases Radiation oncology (London, England)
Baschnagel A, Wolters PL, Camphausen K
2008 Sep 17;3:26. doi: 10.1186/1748-717X-3-26.
-
More
Prognosis for patients with brain metastasis remains poor. Whole brain radiation therapy is the conventional treatment option; it can improve neurological symptoms, prevent and improve tumor associated neurocognitive decline, and prevents death from neurologic causes. In addition to whole brain radiation therapy, stereotactic radiosurgery, neurosurgery and chemotherapy also are used in the management of brain metastases. Radiosensitizers are now currently being investigated as potential treatment options. All of these treatment modalities carry a risk of central nervous system (CNS) toxicity that can lead to neurocognitive impairment in long term survivors. Neuropsychological testing and biomarkers are potential ways of measuring and better understanding CNS toxicity. These tools may help optimize current therapies and develop new treatments for these patients. This article will review the current management of brain metastases, summarize the data on the CNS effects associated with brain metastases and whole brain radiation therapy in these patients, discuss the use of neuropsychological tests as outcome measures in clinical trials evaluating treatments for brain metastases, and give an overview of the potential of biomarker development in brain metastases research.
PMID:18798997 | PMC:PMC2556333 | DOI:10.1186/1748-717X-3-26
View details for PubMedID 18798997
-
More
-
Concurrent capecitabine and upper abdominal radiation therapy is well tolerated Radiation oncology (London, England)
Das P, Wolff RA, Abbruzzese JL, Varadhachary GR, Evans DB, Vauthey JN, Baschnagel A, Delclos ME, Krishnan S, Janjan NA, Crane CH
2006 Oct 24;1:41. doi: 10.1186/1748-717X-1-41.
-
More
We retrospectively evaluated acute toxicity in 88 patients that were treated with capecitabine and concurrent radiotherapy to the upper abdomen. These patients included 28 (32%) with pancreatic adenocarcinoma, 18 (20%) with cholangiocarcinoma, 11 (13%) with ampullary carcinoma, 11 (13%) with other primary tumors, 14 (16%) with liver metastases, and 6 (7%) with metastases at other sites. The median dose of radiotherapy was 45 Gy (range 30-72 Gy). The median dose of capecitabine was 850 mg/m(2) twice daily, with 77% receiving 800-900 mg/m(2) twice daily. The highest grade of acute toxicity was Common Terminology Criteria (CTC) grade 0 in 5 (6%), grade 1 in 60 (68%), grade 2 in 18 (20%), and grade 3 in 5 (6%) patients. No patient had CTC grade 4 toxicity. The most common grade 2 toxicities were nausea, hand-foot syndrome, fatigue, anorexia and diarrhea. The grade 3 toxicities included nausea, vomiting and fatigue. Three patients (3%) required hospitalization due to grade 3 acute toxicity. Capecitabine was interrupted, discontinued or given at an adjusted dose in 13 (15%) patients because of acute toxicity. Therefore, capecitabine and concurrent radiotherapy to the upper abdomen appears to be well tolerated. Capecitabine may serve as an alternative to bolus or infusional 5-FU during chemoradiation for upper gastrointestinal malignancies.
PMID:17062148 | PMC:PMC1634749 | DOI:10.1186/1748-717X-1-41
View details for PubMedID 17062148
-
More
Contact Information
Andrew Baschnagel, MD
600 Highland Avenue, L7/B38,Madison, WI 53792-0001
